Postoperative Radiological Factors Associated with Early Mortality after Decompressive Craniectomy in Acute Subdural Hematoma
Article information
Abstract
Background
Acute subdural hematoma (SDH) often leads to serious neurological deterioration or death. Patients with acute SDH are recommended decompressive craniectomy (DC) if their brain edema is severe. We investigated the association with early mortality through postoperative radiological studies after surgery.
Methods
We retrospectively reviewed 31 out of 85 patients that underwent DC due to acute SDH at our neurosurgical department in January 2011–December 2020. The effect of decompression was estimated through comparison with preoperative and postoperative midline shift (MS) in brain computed tomography (CT). Brain edema was represented as an increased value, measured by comparing the lateral displaced parenchymal diameter with the normal brain diameter.
Results
Of the total 31 patients, 15 died during hospitalization (group A) and 16 had the same or improved neurological status (group B). The reduction rate of MS was shown as higher in group B than in group A; it was significantly different between the two groups. The difference between the two values (DBD) was calculated by measuring the brain diameter of the operative site after DC and normal brain diameter for the progress of brain edema. The difference value of MS (DMS) was greater than DBD for 33.3% and 81.3% of group A and B patients, respectively.
Conclusion
A lower MS reduction rate or higher DBD than DMS increases a patient’s early mortality rate. Therefore, early mortality in acute SDH patients who underwent DC could be predicted through analysis of postoperative brain CT.
INTRODUCTION
Acute subdural hematoma (SDH) is major clinical disease in severe traumatic brain injury (TBI) that often leads to serious neurological deterioration or death. SDH is often associated with intraparenchymal injury and brain swelling in TBI. Surgical treatment is determined by brain imaging study and neurological status; it is recommended by the maximum depth of hematoma and midline shift (MS) 1). The general surgical treatment of SDH is hematoma evacuation. If a brain edema is severe or if it is determined that control of intracranial pressure (ICP) is difficult, a decompressive craniectomy (DC) might be required. Because the cranium is fixed and there is limited anatomical space, ICP will increase as intracranial volume increases due to SDH, which results in decreased cerebral perfusion pressure (CPP). Since patients with acute SDH will worsen over time due to decreased CPP, surgical treatment should be performed as soon as possible.
Despite appropriate surgical treatment and intensive care, the mortality rate for severe SDH with progressive brain edema is high; it is presently 36–79%2). A previous study reported that mortality of SDH patients was related to the Glasgow coma scale (GCS) on arrival, MS and intraoperative brain swelling3). Another study showed the mortality of SDH patients who underwent DC was related to lower GCS, MS, hematoma thickness, and age4). These results show that brain edema is closely related to the prognosis of SDH patients. Therefore, we compared preoperative and postoperative brain computed tomography (CT) and we investigated the association with early mortality through the reduction of MS, aggravation of brain edema and significant findings in postoperative imaging studies.
MATERIALS AND METHODS
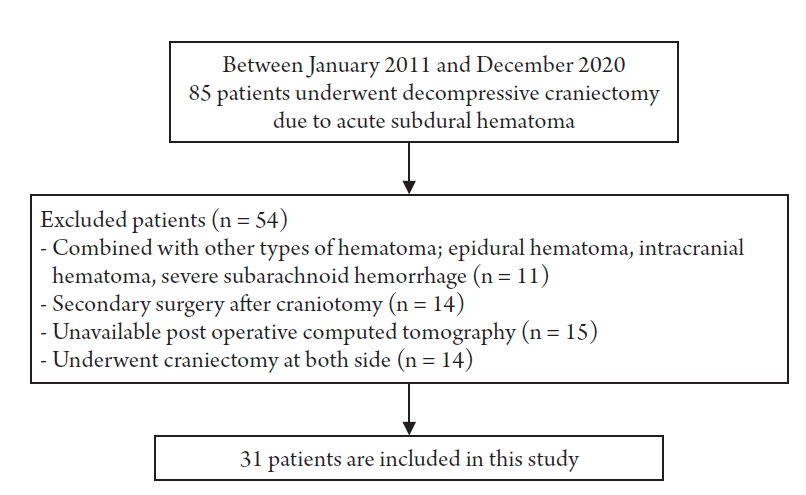
We retrospectively reviewed a total of 85 patients who underwent DC due to acute SDH at our neurosurgical department in January 2011–December 2020. From among these, patients with other types of cerebral hematoma such as epidural hematoma, intracranial hematoma, or severe subarachnoid hemorrhage were excluded. Patients who underwent secondary DC after craniotomy for aggressive of brain edema or rebleeding were excluded. Patients who underwent both DC for both SDH, or for whom postoperative brain CT could not be performed due to unstable vital signs were also excluded (Fig. 1). After exclusion, 31 patients remained who were sorted into two groups. Finally, we analyzed 15 patients who died during hospitalization after DC (group A) and 16 patients who were discharged with the same or improved neurological status at the time of arrival at the hospital (group B). All patients of the group A were expired in 30 days after the operation.
Patients’ age, gender, initial GCS, history of medication including antiplatelet or anticoagulant agents, preoperative pupil response, and time interval from diagnosis to surgery were collected through a retrospective review of medical reports. Preoperative brain CT findings were reviewed and to measure MS with side SDH. MS was determined using the distance from the midline to the septum pellucidum (Fig. 2). Surgical treatment was performed based on indications that an acute subdural hematoma (SDH) with a thickness greater than 10 mm or a midline shift greater than 5 mm on CT scan should be surgically evacuated, regardless of the patient's GCS score. Using the operative records of all patients, intraoperative brain bulging and abrupt hematoma drainage after durotomy were confirmed and all patients underwent duraplasty after hematoma removal. Postoperative brain CT that performed immediately after surgery was also reviewed and the effect of decompression was estimated through comparison with preoperative MS by measuring MS at the same location. To judge the degree of brain edema, the extracranial displaced brain diameter of the craniectomy site was measured based on the septum pellucidum after surgery. Brain edema represented as an increased value when comparing this extracranial displaced brain diameter to normal brain diameter, and the correlation with early outcome was evaluated by comparing this with the reduction of MS (Fig. 3). In addition, the presence of Duret hemorrhage, sulcal effacement of contralateral parenchyma, and intraventricular hemorrhage (IVH) were investigated in postoperative brain CT.

Midline shift (white big arrow) is estimated using the distance from midline (dotted black line) to the septum pellicidum (star) in preoperative computed tomography.

Postoperative midline shift (white big arrow) is estimated in the same way. Difference value of brain diameter (DBD) is calculated by subtracting the normal brain diameter (black arrow) from extracranial displaced brain diameter (white arrow).
Data were analyzed using SPSS software for personal computers (SPSS version 21; IBM Corp., Armonk, NY, USA). Continuous variables were analyzed using the unpaired Student’s t-test and Mann-Whitney U test. Categorical variables were analyzed using the Pearson X2 test and Fisher’s exact test. Variables with statistical significance were selected as predictable values and underwent logistic regression analysis. Any p-value < 0.05 was considered statistically significant.
RESULTS
Of the total 31 patients, 15 (48.4%) died during hospitalization and 16 (51.6%) had the same or improved neurological status. A comparison of 11 variables in total was performed between groups A and B. There was no significant difference in mean age between group A (56.06 ± 15.23) and group B (56.87 ± 13.89), and the patients’ ages were slightly skewed toward elderly in both groups (age, >60 years; odds ratio [OR], 0.889; 95% confidence interval [CI], 0.216–3.662; p = 0.870). There was a higher proportion of male in both groups (p = 0.654). Demographic data showed no significant difference between the two groups. Antiplatelet or anticoagulant drug history with bleeding tendency was higher in group A but there was no significant difference (p = 0.220). Neurological status on arrival at the hospital was defined by GCS score and abnormal pupil response. The initial median GCS score was 4 in group A and 5 in group B; there was no significant difference between the two groups. Abnormal pupil response was defined as pupil size dilatation more than normal (3 mm), negative light reflex, and anisocoria; there was a higher proportion of abnormal pupil response in group A but not to a significant degree (p = 0.333). Overall, group A showed poor neurological status on arrival at the hospital. The time interval from diagnosis to surgery was defined as the difference between the time to examine preoperative brain CT and the time to start anesthesia for surgery. Its median time was 69 minutes in group A and 61 minutes in group B; the difference was significant (Table 1).
We could measure some diameters by brain CT imaging before and after DC. Difference values and reduction rates were calculated by comparing preoperative and postoperative MS. The difference in values of MS (DMS) showed an average of 6.66 ± 2.43 mm in group A and 7.08 ± 2.98mm in group B; it was not significantly different between the two groups (p = 0.668). The reduction rate of MS showed an average 36.20 ± 9.95% decrease in group A and 52.07 ± 19.39% in group B. Most group A patients showed a decrease of ≤50% (93.3%) and there was a significant difference between the two groups (Reduction rate of MS >50%; odds ratio [OR], 0.056; 95% confidence interval [CI], 0.006–0.530; p = 0.006). Then, the difference between the two diameter values (DBD) was calculated by subtracting the normal brain diameter from the extracranial displaced brain diameter; this was significantly different between the two groups (p < 0.001). DMS and DBD were compared and DMS was greater than DBD in 33.3% of group A and 81.3% of group B. More than half of group B patients showed a higher degree of improvement in MS than brain edema; there was a significant difference between the two groups (p = 0.007). Duret hemorrhage after postoperative brain CT occurred at 46.7% only in group A and contralateral sulcal effacement was also confirmed for 46.7% only in group A. Each of these was significantly different (p = 0.002). Postoperative IVH was observed in only 20% in group A; there was no significant difference between the two groups (p = 0.101) (Table 2).
Finally, we confirmed 4 predictable values, Reduction rate of MS > 50%, DMS>DBD, Duret hemorrhage, Contralateral sulcal effacement. Reduction rate of MS > 50% shows higher odds ratio (p=0.050) than DMS>DBD in logistic regression analysis.
DISSCUSSION
Intracranial hemorrhage is a serious consequence of TBI. It can be divided into SDH, epidural hematoma, intraparenchymal hematoma, and subarachnoid hemorrhage by location of hematoma. When TBI occurs, the brain tissue is damaged by primary and secondary injury. Primary injury is described as direct mechanical forces applied to brain tissue; it contributes to secondary brain injury that is defined by dysautoregulation of brain vessels and blood–brain barrier (BBB) disruption5).
SDH represents the largest portion of TBI; patients with SDH had worse outcomes compared to those who had other types of hemorrhage6). When acceleration and deceleration injury occur due to sudden head movements during trauma, separation of the dura layer and arachnoid membrane occurs. This causes rupturing of the bridging vein, which leads to the accumulation of fresh blood in the subdural space; it can be also caused by direct parenchymal contusion or cortical vessel rupture7). This excessive blood volume has a mass effect toward brain parenchyma, causing increased ICP and decreased CPP, which produces additional ischemic injury. Furthermore, disruption of cerebral autoregulation, BBB breakdown, vasospasm and alternation in oxidative metabolism can lead to cerebral blood flow (CBF) decrease and brain edema8-10). The prognosis of SDH has been reviewed in many studies; it is reportedly associated with several factors such as initial GCS, MS, hematoma thickness, pupil response, and basal cistern absence3,4,11-13).
Surgical treatment of acute SDH is recommended if the maximum depth of hematoma and MS are >10 mm and >5 mm, respectively, regardless of the initial GCS. It is also considered if the GCS is <9 points, decreased by 2 points, or there are changes of pupil size1). Surgical techniques include burr hole trephination, craniotomy, and decompressive hemicraniectomy. The main purpose of surgical treatment is to prevent secondary brain injury such as herniation or ischemia by removal of hematoma. It is unclear whether DC is more effective in patients with SDH than craniotomy. Some reported describe that DC shows unfavorable outcomes with surgical complications such as extracranial herniation, subdural effusion, expansion contusion or infection by invasive incision and hydrocephalus14,15). Nevertheless, DC reduces ICP more effectively than other surgical techniques and it leads to the maintenance of adequate cerebral perfusion pressure and improved pressure–volume compensatory reserve16,17). To examine the effectiveness of DC, some studies was reported that surgical groups had a more worse outcomes or showed more severe disability such as vegetative state even though lower mortality18,19). DC has the effect of reducing mortality but is still a challenging procedure in terms of outcome cost-effectiveness. Although there is no definite surgical indication of DC, if severe brain swelling and bulge showed during surgery for SDH, the surgeon should consider DC because it is useful to control ICP.
When SDH occurs, the mass effect of the hematoma itself and progressive brain edema can result in brain displacement. The degree of displacement can be measured by MS on brain CT, which could be a prognostic factor20,21). Some previous studies have shown that MS and hematoma thickness on brain CT in SDH patients were measured and used for prognostic analysis, and patients with MS >3 mm than hematoma thickness had worse outcomes22,23). Since the purpose of DC in SDH patients is to reduce ICP, the effect of DC can be estimated by comparing the MS of preoperative and postoperative brain CT after hematoma removal. In addition, the degree of brain edema can be estimated by extracranial displaced diameter and improvement of MS after DC24). It shows that the changes in MS and brain edema measures after DC were related to early mortality in our study.
Duret hemorrhage has two controversial pathogeneses, but both are caused by increased ICP. The first is arterial origin where a descending transtentorial herniation leads to stretching, spasm, and infarction of central perforating arteries. The other is vascular thrombosis of brain stem due to prolonged elevation in ICP. Duret hemorrhage is commonly associated with severe brain edema and is regarded as a poor outcome25). Sulcal effacement is a result of mass effect as brain edema, the mass effect can push the adjacent gyri together26). Contralateral sulcal effacement could be caused by diffuse brain edema, or ipsilateral brain edema that has a mass effect to the contralateral hemisphere. Therefore, contralateral sulcal effacement seems to be a sign of severe or progressive brain edema. All patients with Duret hemorrhage or contralateral sulcal effacement in postoperative brain CT also had unfavorable outcomes in our study.
There are some limitations in our study. It had a small number of patients, so multivariate analysis is not meaningful. Since this study is a retrospective analysis of a single center, selection bias may have occurred. Further studies should include more patients and be conducted at a multicenter.
CONCLUSION
DC has been performed to control ICP in acute SDH patients. In this study, specific findings at postoperative brain CT are significantly correlated with early outcomes. It has been shown that the lower MS reduction rate or the higher DBD than DMS increases patients’ early mortality rate. The occurrence of Duret hemorrhage or contralateral sulcal effacement was also found to be related to mortality. Then, early mortality of acute SDH patients who underwent DC could be predicted through analysis of postoperative brain CT; this will be useful when discussing patient's treatment plans with guardians.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.