Early In-hospital Management of Acute Ischemic Stroke
Article information
Abstract
Acute ischemic stroke is one of leading causes of disability worldwide. Current therapeutic modalities have very narrow time window, therefore adequate diagnosis and therapeutic modality choice is very important. This article highlights the issues regarding current recommendation of early in-hospital management of acute ischemic stroke. By understanding current recommendation of treatment, we can better access and treat patients with acute ischemic stroke.
INTRODUCTION
Acute ischemic stroke(AIS) is one of the most frequent causes of disability and death worldwide6,16), and enormous social and financial cost due to rehabilitation, long-term care, and loss of productivity17). Leading-therapeutic modalities, include intravenous(IV) thrombolysis and mechanical thrombectomy is extremely time-dependent. Therefore, proper early diagnosis and management is very important. As the reason above, pre-hospital stroke service is one of major concerns of treatment, includes emergency medical services(EMS) and pre-stroke education13,18). Distance and travel time to the nearest stroke center is a crucial issue for time-sensitive stroke treatment5,13).
As mentioned above, current therapeutic modalities have very narrow time window. The phrase “time is brain”8) presents the importance of urgent medical service for patients with stroke. However, as clinical data accumulated, this narrow time window has been expanded and therapeutic modalities have been changed over periods1,15,22,28), although primary “time is brain” motto remains unchanged. In this article, the focus is on the current in-hospital management recommendation of diagnosis of AIS and early therapeutic modalities with review of modification from previous guidelines, helps improving clinical outcomes of stroke patients.
EARLY IN-HOSPITAL MANAGEMENT OF ACUTE ISCHEMIC STROKE
Evaluation
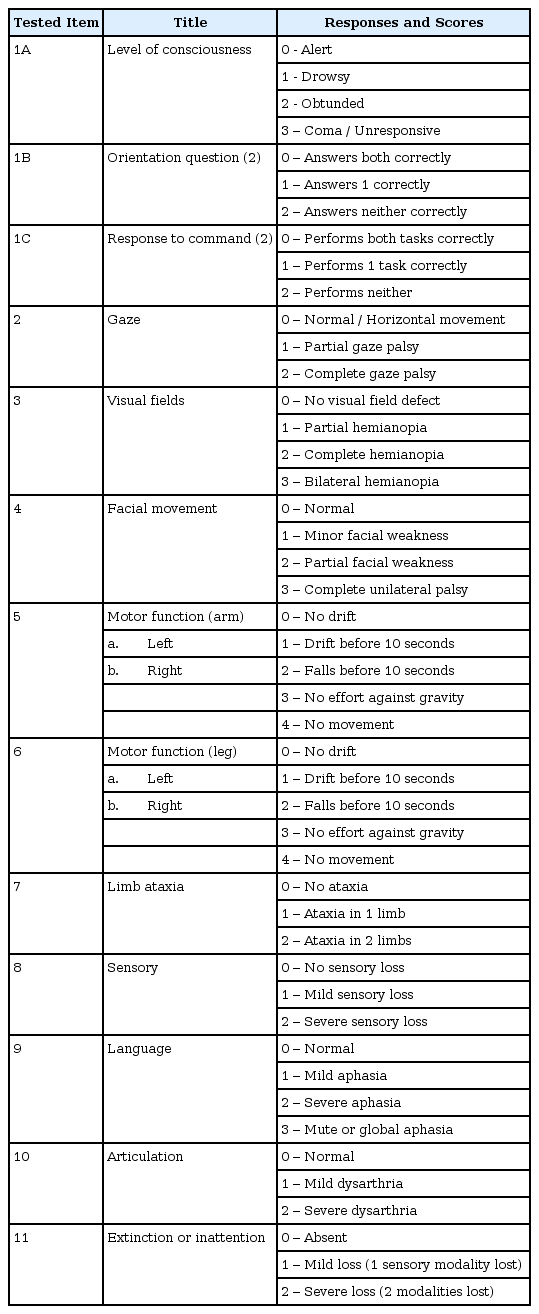
After patient’s arrival on emergency room, use of standardized scale quantifies the degree of neurological deficit, facilitates communication, helps identify patients for thrombolytic intervention is required18). Most preferred rating scale is National Institutes of Health Stroke Scale12) (NIHSS, Table 1). Score represents severity of stoke; 0 : no stroke symptom ; 1-4 : minor stroke ; 5-15 : moderate stroke ; 16-20 : moderate to severe stroke ; 21 -42 : severe stroke.
After exam of NIHSS, proper brain imaging should be followed. All patients admitted to hospital with suspected acute stroke should receive brain imaging evaluation on arrival18). In most cases, non-contrast computed tomography(NCCT) is recommended2,18,21). Although diffusion-weighted magnetic resonance imaging(DW-MRI) is more sensitive than NCCT for detecting AIS3,11), routine use of DW-MRI is not recommended due to the matter of cost-effectiveness18,24). In many cases, diagnosis of AIS is confirmed basis of clinical presentation either a negative NCCT (or show early ischemic change). DW-MRI is recommended when NCCT is negative and patients shows puzzling clinical presentation or uncertain clinical stroke localization18). Powers et al. recommends imaging study should be performed within 20 minutes14,18) of arrival in ER. In some institutions, DW-MRI is routinely performed before IV alteplase administration even if diagnosis of acute stroke was established with NCCT and clinical presentation. This overlapped imaging study is no longer recommended. Also, multi-modal CT and MRI, including perfusion imaging, should not delay followed treatment.
About laboratory data obtaining from blood test, only the assessment of blood glucose is essential18). Other tests include international normalized ratio(INR), activated partial thromboplastin time(aPTT), and platelet count, may be necessary in some circumstances if there is suspicion of coagulopathy. Given the extremely low risk of unsuspected abnormal platelet counts or coagulation studies in a population, IV alteplase treatment should not be delayed while waiting for hematologic or coagulation testing result if there is no reason to suspect an abnormal test. Baseline troponin and electrocardiogram assessment is recommended, but should not delay administration of IV alteplase.
General medical supportive care
Proper airway support and ventilator assistance are recommended for the patients have decreased consciousness. The goal of airway support is supplement of oxygen >94%18,19) of oxygen saturation. But, supplement of oxygen in non-hypoxic patients and any hyperbaric oxygen is not required19).
Optimal blood pressure level ensure best outcome is uncertain23,25). However, Powers et al.18) recommends hypotension and hypovolemia should be corrected to maintain systemic perfusion. Patients who have elevated BP and eligible for treatment with IV alteplase, should their BP carefully lowered to systolic BP <185 mmHg and diastolic BP <110 before IV fibrinolytic therapy is initiated, because the risk of hemorrhage after administration of alteplase is greater in patients with higher BP and in patients with more BP variability. Current recommendation includes maintain BP <180/105 mmHg after 24 hours of treatment.
Source of hyperthermia (temperature >38°C) should be identified and treated20). Hypoglycemia (blood glucose <60 mg/dL) should be treated in patients with acute stroke18).
Intravenous alteplase
After the introduction of IV thrombolysis for the treatment of acute stroke in the 1990s10,28), IV thrombolysis is medical treatment of choice of AIS. IV alteplase (0.9 mg/kg, maximum dose 90 mg over 60 minutes with initial 10% of dose given as bolus over 1 minute) is recommended for standard treatment of AIS. Patients treated with IV alteplase should admit to stroke unit or intensive care unit for close monitoring. Measuring BP and perform neurological exam every 15 minutes during and after IV alteplase administration for 2 hours, then every 30 minutes for 6 hours, then hourly until 24 hours after IV alteplase treatment. During and after IV alteplase administration, delaying placement of nasogastric tube or indwelling bladder catheter is recommended if possible. Patients who treated within 3 hours of symptom onset or last known well time and medically eligible should be treated with IV alteplase. More importantly, exclusion criteria are strictly applied to patients. Contraindications of IV alteplase treatment18); (1) patients who have an unclear time and/or unwitnessed symptom onset and whom the time last known well is > 3 hours or 4.5 hours (2) CT image reveals intracranial hemorrhage (3) prior ischmic stroke within 3 months (4) severe head trauma within 3 months (5) intracranial/spinal surgery within 3 months (6) history of intracranial hemorrhage (7) GI malignancy or GI bleed within 21 days (8) coagulopathy (9) treatment with low-molecular-weight heparin(LMWH) within previous 24 hours (10) infective endocarditis (11) aortic arch dissection. Patients with other medical status including treatment with thrombin inhibitor, glycoprotein IIb/IIIa receptor inhibitor, intra-axial intracranial neoplasm is also potentially harmful to treat with IV alteplase.
In patients eligible for IV alteplase, benefit of therapy is time dependent, and treatment should be initiated as quickly as possible. Even if endovascular thrombectomy is considered, patients should receive IV alteplase therapy if eligible for criteria18).
In former times, IV alterplase administration is applied to patients who treated within 3 hours of onset of stroke symptom or last known well time, because it’s benefit and safety is not well established for patients treated over 3 hours of onset9,27). However, Hacke et al.9) describes benefit of IV alteplase administration to patients treated between 3 to 4.5 hours after onset. Current recommends IV alterplase treatment for selective patients who treated within 3 to 4.5-hour-window18). Resultingly, more patients should be treated with IV alterplase. The benefit of IV alteplase between 3 and 4.5 hours from symptom onset for patients with very severe stroke symptoms (NIHSS > 25) is uncertain, other patients with NIHSS < 25 should treat with IV alteplase in the concern of risks against possible benefits.
The most feared complication of this therapy is symptomatic intracerebral hemorrhage, which occurs up to 7% of treated patients7). Therefore, physician should be prepared for such complication. Management of symptomatic intracranial bleeding during or after IV alteplase is18); (1) stop alteplase infusion (2) hematologic evaluation includes complete blood cell clount(CBC), prothrombin time(PT), INR, aPTT, fibrinogen level, type and cross-match (3) emergent head NCCT (4) cryoprecipitate 10 unit infused over 10-30minutes (5) tranexamic acid 1000mg IV infused over 10 minutes (6) hematology and neurosurgery consultation (6) general supportive therapy.
Mechanical thrombectomy
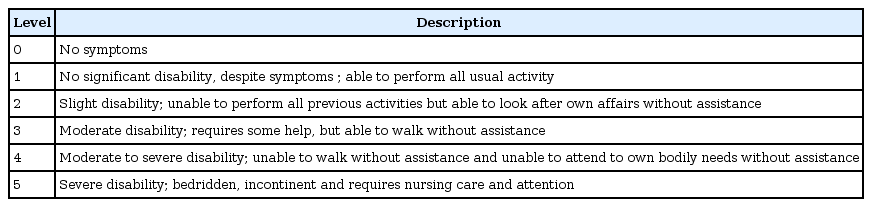
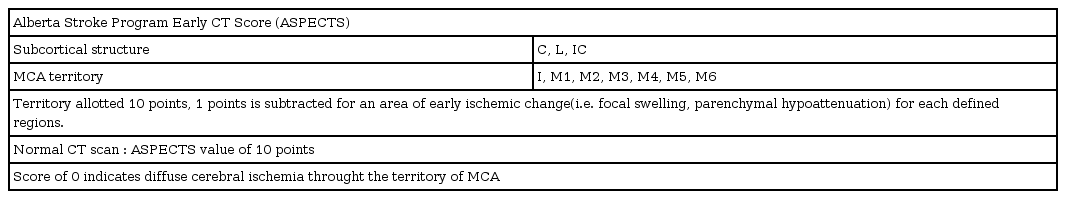
Endovascular treatment(EVT) could provide recanalization of occlusion, and likely to improve clinical outcome28). There are many techniques to achieve removal of clot, mechanical thrombectomy with stent retriever is recommended as the first-choice18). Mechanical thrombectomy is recommended when patients meet all of following; (1) pre-stroke modified Rankin Scale4) (mRS, Table 2) score of 0 to 1; (2) causative occlusion of the internal carotid artery or MCA segment 1 (M1); (3) age ≥18 years; (4) NIHSS score of ≥6; (5) Alberta stroke program early CT score2) (ASPECTS, Fig. 1, Table 3) of ≥6; and (6) treatment can be initiated within 6 hours of symptom onset. Goal of mechanical thrombectomy should be reperfusion to modified Thrombolysis in Cerebral Infarction26) (mTICI, Table 4) grade 2b/3 angiographic result.

Alberta stroke program early CT score (ASPECTS).
C: caudate, I: insula ribbon, L: lentiform, IC: internal capsule, A: anterior circulation, P: posterior circulation, M1–6: middle cerebral artery territory.
Recent two randomized-controlled studies(RCTs) suggest that further expanded time window for mechanical thrombectomy1,15). These RCTs demonstrate usefulness of newer imaging techniques (i.e. CT perfusion) for finding potentially salvageable region(penumbra)28). Eligibility of DEFUSE 3 trial1); (1) initial infarct volume (ischemic core) of less than 70ml; (2) ratio of volume of ischemic tissue to initial infarct volume of 1.8 or more; (3) absolute volume of potentially reversible ischemia (penumbra) of 15 ml or more, and eligibility of DAWN trial15); (1) age ≥ 80, NIHSS ≥ 10, infarct volume < 21 ml; (2) age < 80, NIHSS ≥ 10, infarct volume < 31 ml; (3) age < 80, NIHSS ≥ 20, infarct volume 31ml to < 51 ml. If patients meet these criteria, mechanical thrombectomy is recommended to patients with AIS within 6 to 16 or 24 hours18).
Antiplatelet agent
Administration of antiplatelet agent (i.e. Aspirin) is recommended in patients with AIS within 24 to 48 hours after onset. In former times, initial dose of 325 mg of aspirin is recommended. However, Powers et al. recommends 160 to 300 mg of dosage currently18).
SUMMARY
AIS is one of leading causes of disability and death, and patients suffer long-term care and rehabilitation. As described in article, although therapeutic time window has been expanded through decades, therapeutic time window is still narrow, and benefit from treatment is extremely time dependent. Adequate patient selection and urgent initiation of treatments are essential.
Notes
No potential conflict of interest relevant to this article was reported.