Do Statins Help Prevent VTE After Hemorrhagic Stroke During the Acute Period?
Article information
Abstract
Objectives
The effect of statins on venous thromboembolism (VTE) especially pulmonary embolism (PE) is debatable. This study investigated whether statin medication can decrease the occurrence of VTE in patients with hemorrhagic stroke during the acute period.
Methods
This is a retrospective study of patients with hemorrhagic stroke between March 2011 and December 2013. Patients with newly diagnosed hemorrhagic stroke were observed during 6 weeks of hospitalisation. Occurrence determined using Doppler ultrasound and computed angiography was used to assess risk factors of VTE in patients with hemorrhagic stroke during the acute period. The difference and incidence of the VTE among acute hemorrhagic stroke was analysed in patients who did not receive no statin medication, who received statin medication after the current hemorrhagic stroke, and who received statin medication after previous hemorrhagic stroke.
Results
Among 98 patients, 9 (9.2%) patients representing 3 from each group had VTE (6 for deep vein thrombosis and 3 for PE) during the follow-up. Each incidence of VTE was 6.4%, 13.6%, and 10.3% in patients who did not receive statins, who received statin medication after the current hemorrhagic stroke, and who received statin medication after previous hemorrhagic stroke, respectively (p>0.05).
Conclusions
Moderate instensity dose of statin use was not associated with a reduced risk of VTE in patients with hemorrhagic stroke, especially in the acute period. Randomized, placebo-controlled trials are needed to evaluate the potential benefits of lipid-lowering statin in the prevention of venous thromboembolisn in patients with heamorrhagic stroke.
INTRODUCTION
Venous thromboembolism (VTE) is a common disease affecting more than one per 1000 persons each year17). Patients with hemorrhagic stroke have a relatively high risk of VTE because of immobility and increased prothrombotic activity11). Current guidelines recommend pharmacologic prophylaxis using heparin or low-molecular heparin (LMWH). However many neurosurgeon and neurologist worried about intracerabral hematoma expansion when.
Statins represent a mainstay in the prevention of arterial events. However, two recent meta-analyses suggested that statins may also reduce the incidence of VTE by 10-20%8,13). The ability to decrease tissue factor expression offers a biological rational for statin’s anti-thrombotic effect15). These studies showed that statin medication for more than 3 months helps prevent VTE13,15).
The influence of statins in preventing deep vein thrombosis (DVT) in the acute period could not be explained by these studies since DVT typically develops 2 to 7 days after stroke onset, with about 80% of all DVTs occurring within the first 10 days following a stroke3,16). In cochrane database systematic review in 2014, rosuvastatin was associated with a reduced incidence of VTE although the available date were limited.
The incidence of deep vein thrombosis (DVT) during the first two weeks after stroke ranges from 10% to 75% in untreated patients19,21). Pulmonary embolism (PE) is the most common cause of death between the first two and four weeks after AIS20).
We hypothesised that statin therapy may have a role in VTE prevention in patients with hemorrhagic stroke. We conducted this study to explore the association between moderate intensity statin use and VTE in patients with hemorrhagic stroke during the acute period.
METHODS
Study population and data collection
This retrospective cohort study was at a university-affiliated hospital. The study protocol was approved by the Institutional Review Board of Bucheon St. Mary’s Hospital at the Catholic University of Korea (No. HC13RISE0120). Patients with acute hemorrhagic stroke between March 2011 and December 2013 were enrolled.
Demographic characteristics, risk factors, baseline laboratory results on the day of admission [blood cell count, blood urea nitrogen (BUN)-creatinine (Cr) ratio, osmolarity and C-reactive protein (CRP)], and treatment method data were obtained from the stroke database. The severity of the neurological deficit was evaluated according to the National Institutes of Health Stroke Scale (NIHSSS) in the emergency room. Volume of the intracerebral haematoma was calculated using the formula: ABC/2, where A, B, and C represent the largest dimensions of the haematoma measured at perpendicular angles to one another. Experienced rehabilitation staff checked the severity of weakness by manual muscle test (MMT). MMT results were classified as grade 5 (movement against gravity plus full resistance), grade 4 (movement against gravity plus some resistance), grade 3 (complete range of motion against gravity with no resistance), grade 2 (full or partial range of motion with no gravity), grade 1 (slight contractility without any movement), and grade 0 (no evidence of contractility; complete paralysis).
Inclusion criteria and exclusion criteria
Inclusion criteria were age >18 years, hemorrhagic stroke as confirmed by computed tomography (CT) or magnetic resonance imaging (MRI), onset hemorrhagic stroke within the prior 3 days, and availability for 6 weeks closed observation during hospitalisation. Exclusion criteria were ischaemic stroke; age <18 years; hemorrhagic stroke with vascular anomalies including intracranial aneurysmal rupture, haemorrhage from arteriovenous malformation, or tumorous bleeding; use of anti-platelet medication; discharge or death within 6 week; comorbidities influencing the neurological status, such as seizures or acute respiratory distress; severe stroke (NISSS score ≥20); immobility before stroke onset; and use of hormone replacement therapy.
Diagnosis of VTE and treatment
Doppler ultrasound with the criterion of venous compressibility with transducer and computed DVT angiography were used to diagnose DVT of femoral and popliteal veins. Computed pulmonary angiography with the presence of pulmonary emboli or high probability on ventilation-perfusion scan was used to diagnose pulmonary embolism (PE). All of these diagnostic tools were only used for patients who complained of VTE-suspicious symptoms including pain, tenderness, swelling, warmth, distension of surface veins, Luck’s sign in one or both legs, cyanosis, chest pain, and respiratory distress. Prophylactic use of heparin was not routinely used during the admission period because of concern about hematoma expansion. Thromboembolic deterrent stockings were applied. Early exercise was done 3 days after admission. Patients diagnosed with DVT were treated with full doses of low-molecular-weight heparin. An inferior vena cava filter was also placed, if needed.
Identification of statin use and patient classification
The patients were asked about their current medication including the information on the use of statins. Additionally, medical reports of patients were reviewed to verify the information on the use of statin. Patients were classified into three groups: 1) no statin medication 2) moderate intensity statin use after hemorrhagic stroke, and 3) moderate intensity statin use prior to hemorrhagic stroke.
Moderate stains were defined as atrovastatin 10 mg or 20 mg, rosuvastatin 10 mg and pravastatin 40 mg.
Statistical analyses
Statistical analyses used the Statistical Package for Social Sciences version 18 (SPSS, Chicago, Illinois, USA). Characteristics of hemorrhagic stroke patients with and without VTE were analysed. Variable characteristics were expressed numerically and as percentages. The Mann-Whitney test was used to compare continuous variables like age and body mass index. Fisher’s exact test and chi-squared test were used to compare dichotomous variables including gender, smoking, alcohol, diabetes, hypertension, characteristics of hemorrhage, initial national institute of health stroke scale (NIHSS), severity of muscle weakness, and statin medication. A p value <0.05 was considered statistically significant.
RESULTS
Patients and radiologic findings
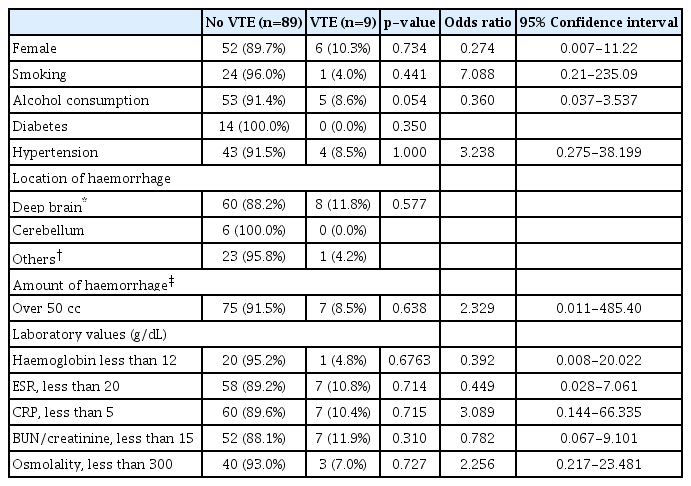
There were 167 patients with acute hemorrhagic stroke. Among them, 98 (40 female and 58 male) patients were included in this study. Of these 98 patients, 9 (9.2%) patients had VTE (6 for DVT and 3 for PE) during the follow-up period. Overall mean age was 59.6 years (range, 19 to 89 years). The mean age of patients with VTE and without VTE was 64 and 59.1 years, respectively (p=0.322). Medical and social conditions such as smoking, alcohol consumption,diabetes and hypertension were evaluated relationship of VTE and PE. There were no relationships. Radiologic finding such as amount of hematoma and location also was not associated development of VTE and PE. Inflammatory marker and dehydration status on admission were no correlation with VTE and PE. The baseline clinical and demographic characteristic of the patients were summarized in Table 1.
Moderate statin use and VTE
Atrovastatin 10 mg (18 patients), atrovastatin 20 mg (5 patients), rosuvastatin 10 mg (14 patinets) and pravastatin 40 mg (14 patinents) were used. Twenty nine patients were taking these statin to hemorrhagic stroke, 22 patients were taken statin after hemorrhagic stroke. The other patients (47 patients) were not taken before and after hemorrhagic stroke. The numbers of patients who had VTE was the same (n=3) in each of the three groups. Moderate intensity statin use was not significantly associated with VTE reduction (Table 2; p=0.539). Also, there was no significant relationship (p=1.000), when patients with acute hemorrhagic stroke were grouped as statin medication and no medication group.
Severity of muscle weakness, active physical therapy
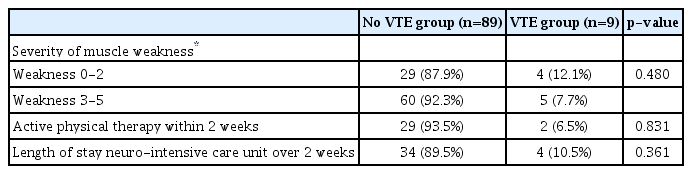
Table 3 shows how severity of muscle weakness influenced prevention of VTE. Thirty three patients had their lower extremities weakness less than grade 2, and 4 (12.1%) patients had VTE, while 5 (7.7%) patients had VTE among 65 patients whose weakness were more than grade 3. These differences were not significant (p=0.480). Also, among 9 patients who had VTE, 7 participated in active physical therapy 2 weeks following hemorrhagic stroke. Only 2 (6.5%) patients had VTE among 31 patients who had received active physical therapy within 2 weeks of hemorrhagic stroke. These differences were not significant (p=0.831). Length of stay in the neuro-intensive care unit was not significant.
DISCUSSION
DVT develops most often between days 2 and 7 after stroke onset; about 80% of all DVTs occur within the first 10 days3,16). DVT was reported in 0.74% patients with ischemic stroke and 1.37% of those with hemorrhagic stroke11). In the International Stroke Trial (IST), 0.8% of patients who did not receive thrombosis prophylaxis developed a clinically apparent PE within 2 weeks after stroke onset7). In one study, PE occurred in 0.51% of ischemic stroke patients and 0.68% of hemorrhagic stroke patients from private hospitals in the United States11). There are few reports on the incidence of PE among Asian stroke patients. The 1997 Chinese Acute Stroke Trial reported PE in 12 (0.1%) of 10,335 ischemic stroke patients who took aspirin for 4 weeks1). Little data concerning hemorrhagic stroke in Asia have been published subsequent to this trial. In our study, 9 (9.2%) patients had VTE (6 for DVT and 3 for PE) during the follow-up period. This incidence was relative low compared with previous study because our study was included only symptomatic patients20). The difference between ischemic and hemorrhagic stroke is the result of less preventive management. DVT can occur about twice as often after hemorrhagic stroke then after ischemic stroke2).
Risk factors of VTE such as older age, obesity, cigarette smoking, hormonal replacement, pregnancy, previous medical illness (such as cancer, COPD, congestive heart failure, thrombophilia, smoking, and hypertension), stroke with limb paresis, and recent surgery were generally accepted22). In recent study, Kim et al proposed that dehydration status in patients with ischemic stroke might be a significant independent risk factor for VTE. In this study, dehydration status, limb weakness and other medical illness were not associated with VTE or PE.
Statins are currently considered the most effective cholesterol-lowering drugs. In addition to lipid-lowering effects, statins have anti-inflammatory effects, reduce serum inflammatory markers, and enhance vascular endothelial function5,14,15). Statins have cholesterol-independent pleiotropic effects that exert vascular protection by improving endothelial function, regulating angiogenesis, stabilising arteriosclerotic plaques, and reducing thrombogenic and inflammatory responses15). Statins also have an antic-coagulation effect by decreasing tissue factor expression, which reduces thrombin generation and attenuates fibrinogen cleavage and factor V/XIII activation5). Thus, statins improve endothelial thromboembolism expression, resulting in increased protein C activation and factor Va inactivation15). Although statins have been associated with a reduced risk of VTE in the general population and ischaemic stroke patients6,8,12,18), their effect on the incidence of VTE in patients with hemorrhagic stroke has not been studied. Presently, the evidence of use of statins in hemorrhagic stroke was not significant. Although all patients were taking an average dose of statins (<80 mg of fluvastatin; <40 mg of lovastatin, simvastatin, or atrovastatin; and <10 mg of rosuvastatin), statin use was not associated with a lowered risk of developing VTE (p=0.539). A recent study reported that statin use was associated with developing VTE and high-dose statin use appeared related to lower occurrence of VTE compared with standard-dose statin use4). However, the latter study concerned the relationship between atherosclerosis and VTE, not hemorrhagic stroke. In our study, only moderate intensity statin (atrovastatin 10 mg or 20 mg, rosuvastatin 10 mg, pravastatin 40 mg) users were included for decreasing side effect of statin. Our study showed that moderate instensity dose of statin use was not associated with a reduced risk of VTE in patients with hemorrhagic stroke, especially in the acute period.
Our study has several limitations. First, the sample size was small and the control group was retrospectively selected only with suspicious symptoms and excluded patients with severe stroke (NIHSS ≥20) or a short hospital stay, regardless of the reason. These reasons lead to inaccurate incidence of VTE and PE in patients with heamorrhagic stroke. Second there was a lack of uniformity in the thromboprophylaxis administered to the patients. Lastly, we could not study the relationship between VTE and statin dosage since all patients receiving an average dose. We did not consider high doses. These limitations should be taken into consideration and need to be addressed in future studies. Nonetheless, the present findings suggest that statin use, especially moderate intensity doses, in patients with hemorrhagic stroke does not decrease the occurrence and risk of VTE.
Many patients after hemorrhagic stroke had permanat neurologic deficits especially limb weakness because limb weakness and immobilization were powerful risk factors22). Also currently recommended pharmacologic prophylaxis by LMWH or heparin could not use after discharge daily. So we need other pharmacologic prophylaxis in these patients. Information about anti-platelet therapy for the prevention of VTE is scarce. A Cochrane review of two small trials including 133 patients (fewer than 0.3% of the participants included in the overall review) showed that anti-platelet therapy did not reduce the risk of VTE9). However, aspirin in the acute stage of ischaemic stroke is associated with a reduction in recurrent stroke and is not associated with an excess of intracerebral hemorrhage7). Statin plus aspirin can reduce risk of VTE in patients with ovarian cancer10). However, anti-platelet medication to patients with acute hemorrhagic stroke would be criticized for risk of rebleeding. So, we should consider whether we could use anti-platelet medication to patients with acute hemorrhagic stroke for preventing VTE.
CONCLUSION
Moderate-intensity dose of statin use is not associated with a reduced risk of VTE in patients with hemorrhagic stroke in the acute period. Randomized, placebo-controlled trials are needed to evaluate the potential benefits of lipid-lowering statin in the prevention of venous thromboembolisn in patients with heamorrhagic stroke.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study protocol was approved by the Institutional Review Board of the College of Medicine of The Catholic University of Korea (HC14RISI0035).
Author contributions
Jung Hyun Park analyzed the data, and drafted and revised the manuscript. Hoon Kim analyzed the data, and drafted and revised the manuscript for intellectual content. Kwangwook Jo was responsible for conceptualization of study, revision of the manuscript for content, helped with study coordination, and interpretation of data.