|
 |
- Search
| J Neurointensive Care > Volume 6(2); 2023 > Article |
|
Abstract
Background
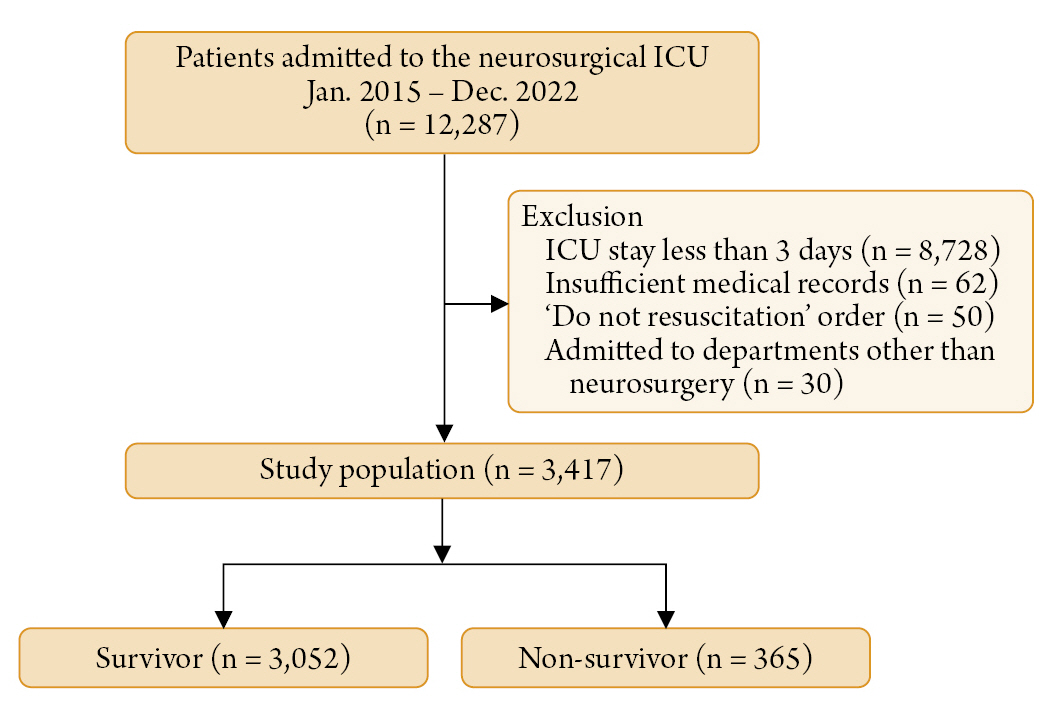
Methods
Results
NOTES
Ethics statement
The study received approval from the Institutional Review Board (IRB) of Samsung Medical Center (No. SMC 2020-09-082) and was carried out in compliance with the principles of the Declaration of Helsinki. Given the retrospective design of the study, the IRB waived the requirement for informed consent.
Author contributions
Conceptualization: JAR. Data curation, Writing – original draft, Writing – review & editing: SC, JAR. Formal analysis, Statistical analysis: SC, HK.
Data availability
Regarding data availability, our data are available on the Harvard Dataverse Network (http://dx.doi.org/10.7910/DVN/ZSFPUY) as recommended.
Acknowledgements
We would like to express our gratitude to Suk Kyung Choo, the nursing director of the neurosurgical intensive care unit, for her valuable advice and insightful discussions. Additionally, we extend our thanks to all the nurses in the neurosurgical intensive care unit at Samsung Medical Center for their exceptional work.
Fig. 2.
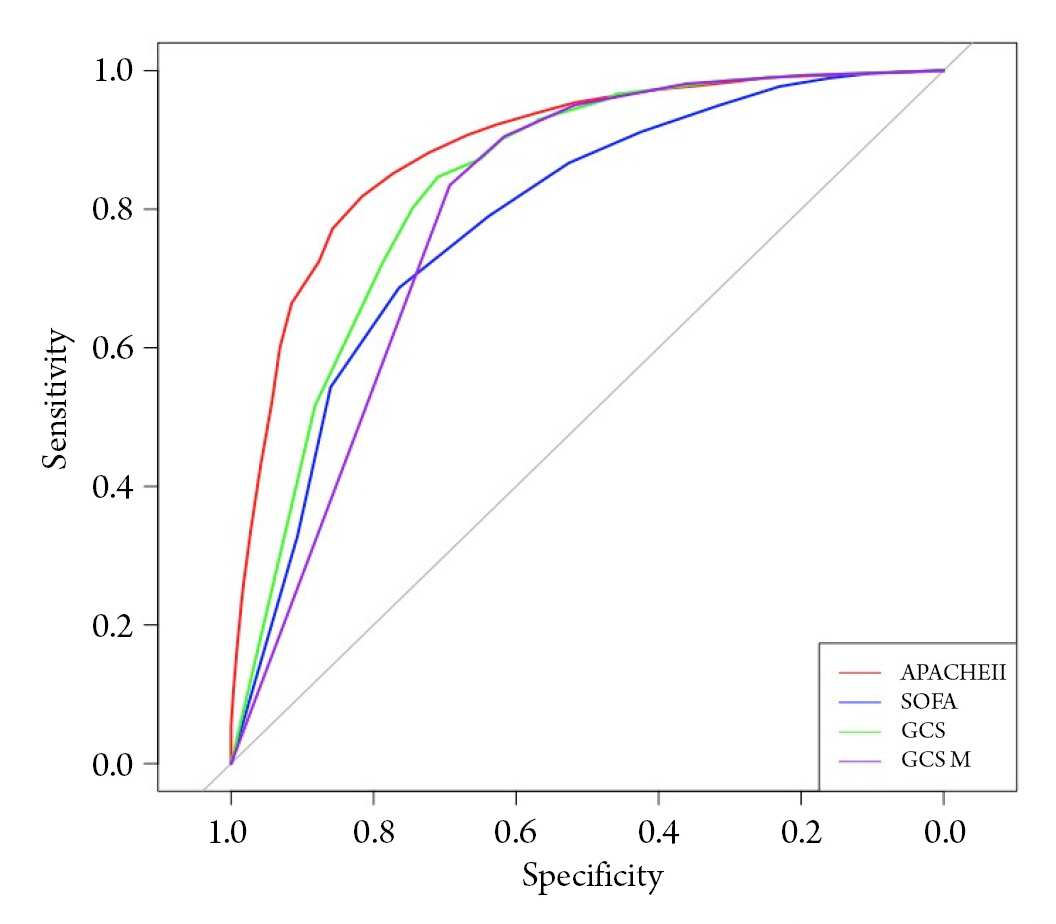
Table 1.
| Survivor (n = 3,052) | Non-survivor (n = 365) | P value | |
|---|---|---|---|
| Patient demographics | |||
| Age (year) | 55.2 (17.0) | 60.7 (16.2) | <0.001 |
| Sex, male | 1587 (52.0) | 192 (52.6) | 0.871 |
| Comorbidities | |||
| Malignancy | 2261 (74.1) | 204 (55.9) | <0.001 |
| Cerebrovascular disease | 871 (28.5) | 188 (51.5) | <0.001 |
| Hypertension | 423 (13.9) | 93 (25.5) | <0.001 |
| Dyslipidemia | 303 (9.9) | 66 (18.1) | <0.001 |
| Diabetes mellitus | 230 (7.5) | 56 (15.3) | <0.001 |
| Chronic kidney disease | 70 (2.3) | 26 (7.1) | <0.001 |
| Chronic liver disease | 52 (1.7) | 16 (4.4) | 0.001 |
| Cardiovascular disease | 45 (1.5) | 12 (3.3) | 0.019 |
| Behavioral risk factors | |||
| Current alcohol consumption | 591 (19.4) | 70 (19.2) | 0.988 |
| Current smoking | 308 (10.1) | 34 (9.3) | 0.708 |
| Primary disease of ICU admission | <0.001 | ||
| Brain tumor | 1983 (65.0) | 140 (38.4) | |
| Malignant brain tumor | 733 (24.0) | 29 (7.9) | |
| Benign brain tumor | 494 (16.2) | 17 (4.7) | |
| Brain metastasis | 704 (23.1) | 76 (20.8) | |
| Hematolymphoid tumor | 52 (1.7) | 18 (4.9) | |
| Intracerebral hemorrhage | 217 (7.1) | 61 (16.7) | |
| Traumatic brain injury | 193 (6.3) | 60 (16.4) | |
| Subarachnoid hemorrhage | 201 (6.6) | 43 (11.8) | |
| Elective vascular surgery | 172 (5.6) | 14 (3.8) | |
| Cerebral infarction | 99 (3.2) | 12 (3.3) | |
| Central nervous system infection | 45 (1.5) | 13 (3.6) | |
| Epilepsy | 28 (0.9) | 1 (0.3) | |
| Others | 114 (3.7) | 21 (5.8) | |
| Cause of ICU admission | 0.036 | ||
| Post-operative management | 1955 (64.1) | 202 (55.3) | |
| Neuromonitoring | 846 (27.7) | 129 (35.3) | |
| Respiratory failure | 61 (2.0) | 10 (2.7) | |
| Pre-operative management | 27 (0.9) | 4 (1.1) | |
| Cardiovascular disease | 14 (0.5) | 2 (0.5) | |
| Postcardiac arrest syndrome | 14 (0.5) | 0 (0.0) | |
| Post-procedural management | 11 (0.4) | 3 (0.8) | |
| Others | 124 (4.1) | 15 (4.1) | |
| ICU management | |||
| Mechanical ventilation | 1045 (34.2) | 305 (83.6) | <0.001 |
| Continuous renal replacement therapy | 29 (1.0) | 28 (7.7) | <0.001 |
| ICP monitoring | 745 (24.4) | 107 (29.3) | 0.047 |
| Use of mannitol* | 2294 (75.2) | 248 (67.9) | 0.003 |
| Use of glycerin* | 825 (27.0) | 188 (51.5) | <0.001 |
| Use of more than one hyperosmolar agent | 2309 (75.7) | 258 (70.7) | 0.044 |
| Use of vasopressors and inotropic agent | 24 (0.8) | 21 (5.8) | <0.001 |
Table 2.
| Survivor | Non-survivor | p value | |
|---|---|---|---|
| APACHE II score | 11.22 ± 5.22 | 21.70 ± 6.78 | <0.001 |
| SOFA score | 1.97 ± 2.18 | 5.10 ± 3.38 | <0.001 |
| GCS | 13.42 ± 2.64 | 8.05 ± 4.43 | <0.001 |
| GCS M | 5.66 ± 0.88 | 3.61 ± 1.94 | <0.001 |
Table 3.
| Adjusted Odds Ratioa (95% CI) | p value | |
|---|---|---|
| APACHE II score | 1.19 (1.15 – 1.23) | <0.001 |
| SOFA score | 1.13 (1.07 – 1.19) | <0.001 |
| GCS M | 0.72 (0.64 – 0.81) | <0.001 |
| Malignancy | 1.47 (1.09 – 2.01) | 0.014 |
| Mechanical ventilation | 1.92 (1.33 – 2.80) | 0.001 |
| Continuous renal replacement therapy | 2.79 (1.38 – 5.61) | 0.004 |
| ICP monitoring | 0.61 (0.44 – 0.84) | 0.003 |
REFERENCES
- TOOLS