Cerebellar Stroke: A Primer on Diagnostic Considerations and Therapeutic Options
Article information
Abstract
Cerebellar infarction is an infrequent pathology that represents only 3% of ischemic strokes with associated high morbidity and mortality. Immediate medical and surgical interventions are required for early and timely clinical management; making correct use of diagnostic images, the start of support measures that allow patient stabilization, and adequate surgical treatment to counteract the progression to catastrophic herniation.
INTRODUCTION
Cerebellar infarction accounts for 3% of all ischemic strokes in the United States, with 27,400 new cases diagnosed each year1,2). This pathology, although infrequent, has serious complications and repercussions. This is considered to be the most common cause of vascular vertigo3-5). This was first described in 1956 by Fairburne et al. as a pathology that compromised the circulation of the posterior fossa with edema and compression of the brain stem6). This pathology is mainly caused by the occlusion of the vascular flow or trauma to 3 main arteries of the vertebrobasilar system: the posterior inferior cerebellar artery (PICA-which is usually more frequently associated with this pathology), the anterior inferior cerebellar artery (AICA) and superior cerebellar artery (SCA)2,5,7). The infarction in this vascular system generates edema related to ischemia and metabolic changes that modify the functioning of the tissue, thus leading to cellular swelling secondary to energy failure. This process causes the posterior fossa to undergo a mass effect caused by the edema ultimately obstructive hydrocephalus and herniation. This hydrocephalus is due to blockage of cerebrospinal fluid (CSF) passage in the fourth ventricle or the aqueduct of Sylvius, the latter is one of the greatest complications and leads to high mortality and morbidity2,5).
This pathology usually manifests with vertigo, nausea, nystagmus, vomiting, uncoordinated gait, and headache. There are some important clinical characteristics: site-manifestation correlation, where, for example, Wallenberg syndrome or lateral spinal cord syndrome associated with PICA alteration, Foville syndrome with AICA alteration, and Mill syndrome with SCA alteration2,5,7).
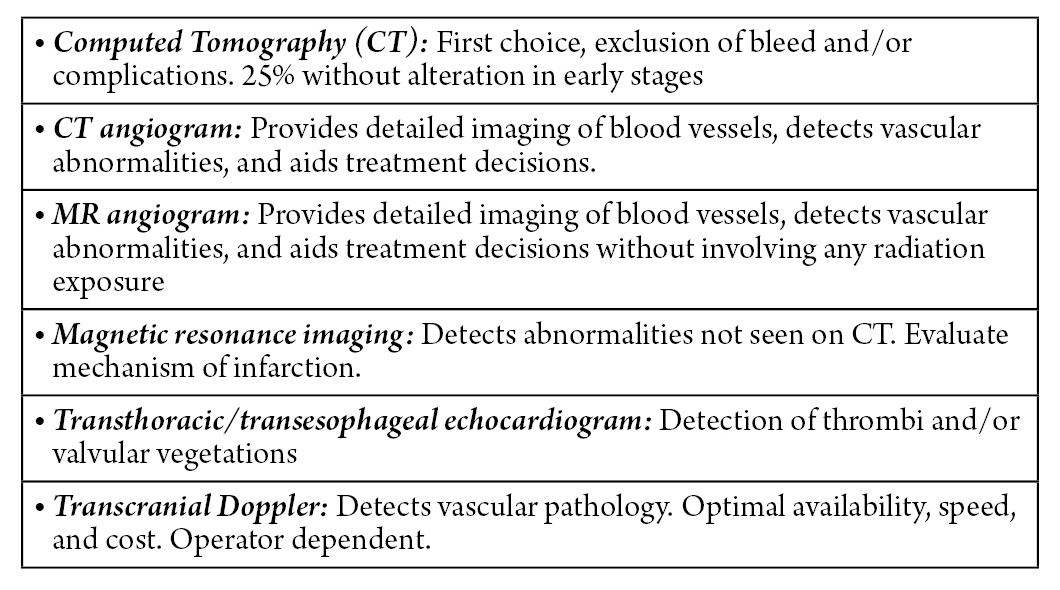
DIAGNOSTIC CONSIDERATIONS
Given the clinical suspicion of a cerebellar ischemic condition, an evaluation is carried out using brain images (Fig. 1)8). Computed axial tomography (CAT) without contrast of the skull is the first-line diagnostic test in the evaluation of patients with clinical suspicion of cerebellar infarction. It allows the exclusion of the presence of posterior fossa hemorrhage, and possible complications inherent to the infarction such as obstructive hydrocephalus. However, more than 25% of patients can have a normal computed tomography (CT) in the initial period1,9). CT scan of the brain in a stroke patient is usually done with a CT angiogram and CT perfusion. With a high clinical suspicion and early diagnosis of this stroke, a magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) of the brain without contrast is deemed prudent.
The CT angiogram of the head and neck allows the cause of the ischemic event to be identified, such as dissection or embolic occlusion, as well as looking for basilar artery occlusion. Compared to CT angiogram, head and neck magnetic resonance angiography provides better detail of the vasculature in the setting of severe blood vessel calcification10). MRI of the head detects abnormalities not identified by CT and provides details about the mechanism of infarction. However, its disadvantages include the longer time required to perform it, the risk of the patient’s airway crisis, and the impossibility of its use in patients with electrical or metallic devices1).
The transthoracic echocardiogram and the transesophageal echocardiogram, as diagnostic modalities, provide information about cardiac valvular vegetations or thrombi as a cause of posterior circulation embolic occlusions1).
Transcranial doppler (TCD) allows evaluation of the existence of underlying vascular pathology and/or abnormality with the advantages in terms of availability, rapid speed, and lower monetary cost. The only major limitation is that the quality of testing is operator-dependent2).
Differential diagnosis
The differential diagnosis for cerebral ischemic stroke-like symptoms includes meningitis or other infectious diseases, syncope, neoplasm, seizure, hypoglycemia, cerebral hemorrhage, and electrolyte imbalance.
SUPPORT MEASURES AND INITIAL MANAGEMENT
The guidelines for the management and initial treatment in the context of the patient with cerebellar infarction are not specific exclusively for the cerebellar condition due to the lack of randomized trials and evidence in the scientific literature, which is why its management results from the existing information about acute cerebrovascular accident (CVA) in general. The “American Heart Association” (AHA) in its latest update on early stroke management provided a set of recommendations on general supportive care and emergency treatment2,8).
Airway and oxygenation
Airway and ventilatory support are indicated in the context of a decreased state of consciousness and/or alteration at the bulbar level that can compromise the respiratory mechanism. Likewise, it is important to maintain oxygen saturation above 94% with the use of supplemental oxygen8). However, supplemental oxygen is not recommended in patients who do not present with hypoxia, likewise, the use of hyperbaric oxygen is not indicated unless the cause is air embolization1,8).
Blood pressure
The range of blood pressure (BP) values to maintain is not yet established, however, it is important to correct hypotension and hypovolemia by reaching blood pressure levels that guarantee optimal tissue perfusion and adequate organ function2,8). A study by Wohlfahrt et al. found that patients with mean arterial pressure (MAP) less than 100 mmHg at admission had a higher risk of death than those with MAP between 100–110 and 110–121 mmHg. Similarly, a systolic blood pressure (SBP) lower than 120 mmHg showed a higher risk of death compared to patients with SBP between 120–130 and 130–141 mmHg. There is currently no evidence to guide the amount and duration of intravenous fluids8,11).
Body temperature
Temperature elevation greater than 38°C or hyperthermia should be duly recorded and treated with antipyretic medications until a state of normothermia is reached. However, there is currently insufficient evidence to recommend the induction of hypothermia, which despite being known as a neuroprotective strategy is associated with an increase in infections1,8,12).
Blood glucose
Hyperglycemia within the first 24 hours in the context of a cerebrovascular accident (CVA) is associated with worse results and clinical outcomes1). Therefore, it is important to maintain blood glucose between 140 and 180 mg/dL and constant monitoring to avoid a state of hypoglycemia8).
Fibrinolytic therapy
The use of intravenous alteplase is recommended at a dose of 0.9 mg/kg (maximum dose of 90 mg) over 60 minutes, administering an initial bolus of 10% of the dose in 1 minute, within 3 hours of the onset of symptoms of stroke, and even within 3 to 4.5 hours according to the criteria established by the AHA8,13). Continuous observation during fibrinolytic therapy is essential because of possible adverse effects, such as hemorrhagic complications and angioedema that would lead to partial obstruction of the airway. In the scenario of patients with high BP and with eligibility criteria for treatment with alteplase, an SBP of less than 185 mmHg and a diastolic BP (DBP) of less than 110 mmHg are recommended before starting fibrinolytic therapy8). Evidence of cerebral microbleeds (>10) on MRI and the initiation of alteplase treatment may be associated with an increased risk of symptomatic intracerebral hemorrhage8).
Antiplatelet and anticoagulant therapy
Within the first 24 and 48 hours, administration of aspirin is indicated, however, in patients treated with alteplase/tenecteplase (TNK) (Table 1), its administration can be delayed up to 24 hours later. Treatment with dual antiplatelet therapy- aspirin and clopidogrel- started within the first 24 hours and continued for 21 days contributes substantially to the early prevention of secondary stroke, even up to 90 days after the episode. Anticoagulant therapy for the prevention of another ischemic attack or to improve the clinical condition of the patient is not recommended8).
For a long time, acute ischemic stroke has been treated using intravenous thrombolytics, with tissue plasminogen activator (TPA) and TNK being the approved agents for the treatment of acute ischemic stroke. TPA is recommended for administration within 3 hours of symptom onset; however, studies suggest that it may be safe and beneficial if given as long as 4.5 hours later14).
The use of TNK in acute stroke is recommended by guidelines from the American Heart Association/American Stroke Association for certain patients who meet specific criteria, such as those with large vessel occlusion and who can receive treatment within 4.5 hours of symptom onset. The EXTEND-IA TNK trial showed early reperfusion in 22% of patients who received TNK versus 10% of those who received alteplase15).
SURGICAL MANAGEMENT
Surgical treatment of cerebellar infarction is external ventricular drainage (EVD), suboccipital decompressive craniotomy (SDC) with dural expansion, or a combination of the two. (two)
Suboccipital decompressive craniotomy
SDC is a safe procedure that is indicated if the initial medical treatment has not been favorable or the patient's condition deteriorates rapidly. The mortality of SDC is between 29.5% and has an 83% chance of not causing mild disability16). The purpose of this is to provide space for the edematous cerebellum, relieving compression of the fourth ventricle and the brainstem. If obstructive hydrocephalus occurs in addition to the cerebellar infarction, management with a ventriculostomy (EVD) added to a SDC is considered1,17). Several studies have indicated that EVD can be performed as initial management followed by SDC if no improvement is seen. However, the time that must elapse between the two procedures, EVD and SDC, has not been defined. Horwitz and Ludolph have given the option of performing SDC after a few hours, evidenced by the deterioration of the neurological condition after EVD placement18).
The neurological signs looked for in patients are: a decreased level of consciousness, downward conjugate gaze or sunset eyes, gaze paresis, cranial nerve deficits, and long tract signs. The outcome has been associated with the patient's preoperative state of consciousness, regardless of the treatment that has been carried out, whether surgical or not1,18).
Among the contraindications of SDC are clinical or radiological signs of severe or irreversible ischemia of the brain stem, severe comorbidity, or refusal to undergo the procedure19).
External ventricular drainage
EVD is part of the first surgical measures that can be implanted in a patient who is stable in the initial stage of the cerebellar infarction, and who consequently has a mass effect secondary to tissue edema or obstruction of the flow of CSF in the posterior fossa, producing hydrocephalus and/or compression of the brain stem5). This therapeutic strategy has shown better results than medical treatment with significant positive results when evaluating neurological improvement with the Glasgow scale.
The EVD has been widely recommended in the literature due to its therapeutic value since with its application, improved inpatient survival20,21). In the literature, the use of EVD is recommended in hydrocephalus or brainstem compression secondary to cerebellar infarction, either as the only intervention or in conjunction with SDC. However, data suggest that EVD should be carried out alone and subsequent SDC if there is no improvement in neurological and clinical parameters found. There have been few studies comparing EVD intervention alone with EVD + SDC therapy, and although these do not show significant differences in survival, these do suggest that there is an improvement in long-term functional parameters1,6-8,18,22-26). However, the possibility of triggering a transtentorial herniation and additionally increased risk of ventriculitis and other neurologic infections have been attributed to EVD, for which clinical evaluation of the patient should be carried out regularly in search of signs and symptoms that may suggest the diagnosis1,2,7,8).
CONCLUSION
Cerebellar infarction is a rare entity with serious consequences for the patient's health. It presents with unspecific clinical symptoms, however, the suspicion of this entity must be considered with signs of cerebellar dysfunction. CT of the head is the first-line image to carry out its imaging diagnosis. Initial management includes airway control, blood pressure, blood glucose, temperature, fibrinolytic, and antiplatelet/anticoagulant therapy. Likewise, surgical measures, such as EVD and SDC, are necessary to resolve complications of this entity.
Notes
Ethics statement
We confirm that, for this work ethical guidelines, ethical approvals (institutional review board) and the use of informed consent were not applicable.
Author contributions
Writing – original draft: NA, GB, S, BOH,CMA, GHD. Writing – review & editing: TJ, MK, LFM.
Conflict of interest
There is no conflict of interest to disclose.
Funding
None.
Data availability
None.
Acknowledgements
None.