Ultrasound-guided placement of Midline catheters performed by Neurosurgery resident as a substitute for conventional central venous catheter placement in Neurosurgical Intensive Care Unit patients
Article information
Abstract
Background
Midline catheters (MCs) and peripherally inserted central venous catheters (PICCs) are placed by a specialist or trained nurse to avoid the complications of conventional central venous catheters (CCVCs). The safety of MC placements by residents has not been evaluated. We investigated the safety of ultrasound-guided MC placement by neurosurgery residents as a substitute for CCVC placement in patients in the neurosurgical intensive care unit (ICU) requiring intravenous (IV) therapy ≤14 days.
Methods
Between July 2022 and June 2023, 57 MCs for 44 patients, 36 CCVCs for 35 patients, and 32 PICCs for 32 patients were placed during their ICU stay. One resident performed all MC placements under ultrasound guidance. The baseline and procedure-related parameters of the three catheter groups (MC, CCVC, and PICC) were analyzed, with focus on the comparison of complications between MC and CCVC.
Results
The first-attempt success rate was significantly higher (p=0.003) in the MC group (96.5%) than in the CCVC group (80%). Insertional injuries occurred more frequently in the CCVC group than in the MC group (13.3% vs 0%, p=0.012). The rate of completion of therapy was significantly higher (p=0.002) in the MC group (86.0%) than in the CCVC group (53.3%). The incidence of bloodstream infections did not differ significantly between the MC and CCVC groups (p=0.605).
Conclusion
MCs placed under ultrasound guidance by neurosurgery residents for neurosurgical ICU patients is safe and may be a substitute for CCVC indicated for IV therapy ≤14 days.
INTRODUCTION
Most patients in neurointensive care need intravenous (IV) maintenance or resuscitation and medications to manage the increased intracranial pressure (IICP) or complications that may occur during hospitalization1). Therefore, establishing IV access, including central venous access, is imperative in these patients. Conventional central venous catheter (CCVC) placement has limitations of catheterization failure, central line-associated bloodstream infection (CLABSI), and mechanical injury, which can be fatal2,3). Mechanical complications occur in 1.5% and 8% of CCVCs and more common among catheter insertions performed by junior practitioners than by experienced physicians4-6). CCVC insertion under ultrasound guidance is safer but may be difficult for inexperienced operators3,7,8). Reduced resident working hours and the lack of easily accessible educational programs can worsen this problem.
A peripherally inserted central venous catheter (PICC) and a midline catheter (MC) can avoid the risk of insertion-related mechanical injury with an acceptable risk of catheter-related bloodstream infection (CRBSI)9,10). PICC is recommended for patients requiring IV therapy > 14 days9). Meanwhile, MC is an emerging device commonly used for short-term (≤ 14 days) IV therapy without a definite need for central venous access9). MCs are placed in a deep vein of the upper arm and the tip of the catheter is located in the axillary vein9). PICCs are also inserted in the same veins but its length is relatively long, sufficient to access the central veins9). Both PICC and MC insertions require surgical aseptic techniques and are usually performed by neurointensivists, specialists, or trained nurses9,11). However, these skilled personnel are not available at every time nor at every hospital. There is insufficient background or evidence for MC placement by neurosurgical residents. This study aimed to determines whether MC placement by residents could be a solution for the complications of CCVC catheterization performed by residents. We investigated the safety and efficacy of ultrasound-guided MC placement by neurosurgery residents in neurosurgical ICU patients who require IV therapy with an expected period of 14 days or less.
METHODS
This retrospective study evaluated adult neurosurgical patients (aged over 18 years) admitted to the neurosurgical ICU of our institution between July 2022 and June 2023. Patients who underwent central venous catheterization, including CCVC and PICC, and/or MC placement during their ICU stay were included. Catheterizations for hemodialysis (HD) were excluded because the purpose of the catheter was wholly different, and anesthesiologist or interventional radiologist placed all HD catheters during the study period. The institutional review board approved this study and the requirement for informed consent was waived because of the retrospective nature of the study.
Electronic medical records, neurosurgical department registry of catheter placements, and radiological data were retrospectively reviewed. Data on demographic characteristics, age, sex, and body mass index (BMI) were collected from the medical records. The Acute Physiology and Chronic Health Evaluation (APACHE) II score at the time of ICU admission, cause of ICU admission, comorbidities, and antithrombotic administration were also collected. Procedure-related characteristics including indications for catheter placement, Glasgow Coma Scale (GCS) score at the time of catheterization, use of ventilator/inotropics, whether the operator was a resident, application of ultrasound guidance, location of catheterization (ICU or others), coagulation study at the time of catheterization, whether the paretic arm was catheterized, veins of accessed and specifications of used catheters were also derived from medical records.
The placements were classified into three groups according to the catheter used (MC, CCVC, and PICC). And the patient characteristics and procedure related parameters were analyzed, with a focus on the comparison between MC and CCVC groups. The primary outcomes were procedure-related parameters including the success of the first attempt, procedure time, and insertion-related injuries. The secondary outcomes were the maintenance of catheters, specified as the completion of therapy, and complications that occurred during IV therapy. Completion of therapy was defined as catheter use untill the end of the intended purpose or for 14 days. Major complications were defined as serious insertional injuries, including pneumothorax, hemothorax, nerve injury, and bleeding, for which management was required beyond sandbag/manual compression. CRBSI, including CLABSI and venous thromboembolism (VTE), were classified as major complications. Diagnosis of CRBSI was made based on the criteria from a previous study: (1) positive peripheral blood culture drawn with the presence of catheter or within 48 hours from the removal of the catheter; (2) detection of the same microorganism on catheter tip culture or a positive culture result of blood drawn via the catheter, which was obtained simultaneously with the peripheral blood sample; and (3) absence of any other suspicious source of bacteremia11-13). If the blood culture was positive, CRBSI was investigated in cooperation with our institution's Infectious Diseases Department and the Infection Prevention and Control Team. VTE was diagnosed by imaging confirmation (bedside ultrasound or computed tomography scan) of symptomatic deep venous thrombosis (DVT) in the relevant extremities or lungs, which was absent at the time of catheterization12). Minor complications were defined as phlebitis, leakage of infusate into the soft tissue, dislodgement, and catheter occlusion. The criteria for minor complications were based on previous studies12,14). Insertion site bleeding as minor complications, that could be sufficiently managed with compression were also analyzed.
One postgraduate year (PGY)-3 resident performed all MC placements under strict surgical aseptic technique during the study period. One neurosurgical specialist (the corresponding author) provided an education program to the resident regarding MC placement using with a traditional method15).
During the study period, the attending specialist selected the type of catheter to be used. However, if more than 2 weeks of IV therapy for any cause was anticipated, PICC was recommended by the operator (corresponding author) as appropriate. A 4-French (Fr) single-lumen Seldipur Smartmidline (Vygon, Ecouen, France) with 12cm length was used in all patients. All punctures were performed in the basilic, or brachial, or cephalic vein under ultrasound guidance and with the Seldinger technique. Meanwhile, CCVCs were placed by neurosurgical residents of PGY 3 and 4 neurosurgical residents, neurosurgical specialists, anesthesiologists, or interventional radiologist. The subclavian, or internal jugular, or femoral veins were accessed for CCVC placement with or without ultrasound guidance. A 7-Fr Arrowg+ard Blue® Two-lumen CVC (Arrow International, Inc., Everett MA, USA), 7-Fr Three-lumen CVC (Arrow International, Inc., Everett MA, USA), 7-Fr Prime-S Antimicrobial Double-lumen (Sungwon Medical, Cheongju, South Korea) or 7-Fr Prime-S Antimicrobial CVC Triple-lumen (Sungwon Medical, Cheongju South Korea) were used. All PICC procedures for neurosurgical patients have been performed by a single operator (corresponding author) with several years of experience in endovascular neurosurgery since 2018 until today. PowerPICC® Catheter 4(5,6) Fr single(dual, triple)-lumen (Bard, Salt Lake City, UT, USA) were used in all PICC cases. All catheter insertion sites were dressed every 7 days or when compromised.
Categorical values were presented as numbers (percentages) and a Fisher’s exact test and chi-square test were used to analyze. Continuous variables were presented as the median (interquartile range [IQR]) and analyzed using the Mann–Whitney U test. All statistical analyses were performed using MedCalc (MedCalc Statistical Software version 19.1.7 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2020). A p value of<0.05 was considered significant.
RESULTS
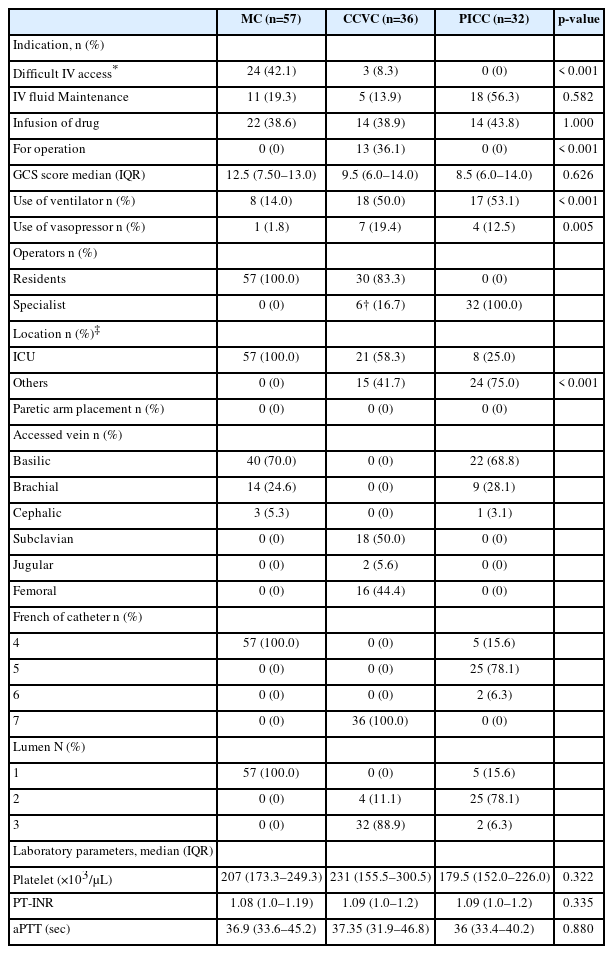
There were 57 MC placements in 44 patients, 36 CCVC placements in 34 patients, and 32 PICC placements in 32 patients admitted to ICU. Considering the patients who received catheter exchange to other types, there were a total of 97 patients in this study. The baseline demographic characteristics of the patients are described in Table 1. Age, sex, BMI, incidence of comorbidities, and antithrombotic use did not show significant differences among the three groups. In total, 51 patients (52.6%) were male. The median age and BMI of the MC group were 75 years (IQR, 55.5-83.0, years) and 22.6 kg/m2 (IQR, 21.3-26.9 kg/m2), respectively. The most common cause of ICU admission was hemorrhagic stroke (53.5%), followed by traumatic brain injury (30.9%). The median APACHE II score was higher in the CCVC group (21.0) than in the other two groups (15.0 and 17.5, p=0.010). The most common comorbidity was hypertension (62%).
MCs were more frequently placed than CCVCs and PICCs in patients with difficult peripheral IV access (p<0.001). Meanwhile, CCVCs were more frequently placed than MCs and PICCs for intraoperative anesthetic management (p<0.001). The rates of ventilator use and vasopressor administration were higher in the CCVC group than in the MC group (p<0.001 and 0.005, respectively). The GCS score, coagulation studies at the time of catheterization were not significantly different among the three groups. The rate of catheter insertion in locations other than the ICU was higher in the CCVC group than in the MC group (p<0.001), and this may reflect the tendency for CCVCs to be placed in the operation room. In the MC group, the basilic vein was the most common access site (70.0%). In the CCVC group, the most common access site was the subclavian vein (50%), followed by the femoral vein (44.4%). No patients underwent MC or PICC placement in the paretic arms (Table 2).
The procedure outcomes are presented in Table 3. Of the 36 CCVC placements, 30 were performed by neurosurgical residents and these were included in the outcome analysis. The first-attempt success rate was significantly higher in the MC group than in the CCVC group (96.5% vs 80%, p=0.003). The ultrasound-guided technique was more frequently adopted in the MC group than in the CCVC group (p<0.001). Insertional injury, limited as a major complication, was more commonly observed in the CCVC group (2 cases of pneumothorax) than in the MC group (n=0), but the difference was not significant (p=0.147). However, when hematoma (1 subclavian and one femoral) managed by 2-3 days of sandbag compression was included in the analysis, the rate of insertional injury was significantly higher in the CCVC group (p=0.012).
There was no significant difference regarding the procedure time between the MC and the CCVC groups (p=0.332). The catheter dwell time was the longest in the PICC group, with a median of 21.0 days (IQR: 15.0-29.0 days). And the median dwell time was longer in the MC group (11.0 days) than in the CCVC group (7.5 days); however, there was no significant difference (p=0.114). The therapy completion rate was higher in the MC group than in the CCVC group (p=0.002). Deaths before completion (n=3), dislodgement (n=2), CRBSI (n=2), and phlebitis (n=1) were the causes of catheter removal before completion of therapy in the MC group. For the CCVC group, all femoral vein catheters were changed to other catheters, even in the absence of complications, because they precluded ambulation, and there was concern that the longer the period of maintenance, the greater the risk of complications. The causes of CCVC removal that did not result from the completion of therapy were CRBSI (n=2), phlebitis (n=2), and dislodgement (n=1). The incidence of CRBSI was higher in the CCVC group (6.7%) than in the MC group (3.5%); however, the difference was not significant (p=0.606). The total complication rate (33.3%), including both major and minor complications, was higher in the CCVC group than in the MC group (p=0.007).
DISCUSSION
CRBSI and catheter - related venous thrombosis in MCs and CCVCs
MC has replaced certain parts of traditional CCVC indications10). Emergence of MC use has attributed to the acceptable incidence of MC-related BSI documented by previous studies. In a systematic review comparing various IV catheters, MC-related infections occurred in 0.4% (0.2/1,000 catheter-days)16). This was lower than the infection rates of central venous (4.4%, 2.7/1,000 catheter-days) catheters16). CRBSI in the MC of our study was 3.5%, slightly higher than that in the above systematic review16). However, this is lower than the 6.7% incidence rate of CCVC-related CLABSI in our study. Although there is no significance, the lower rate of BSI in the MC group is consistent with previous findings.
The first-attempt success rate of MC placement in our study was higher than that reported previously (96.5% vs. 83.4%)12). The number of attempts is important for ensuring the safety of MC maintenance. In a previous study, when the first attempt of MC insertion was achieved, the rate of MC-related venous thrombosis was 4.5%, but when the attempts were repeated three times or more, the venous thrombosis rate was increased up to 9%17). DVT of the upper arm can the potentially cause fatal complications resulting from any IV catheters10). The target vein of the MC is usually smaller than that of central veins. Therefore, MCs have an inherently higher risk of DVT formation in the upper arm than CCVCs18). In our study, no DVT occurred in any of the catheter groups. The high first-success rate in our study may be attributable this favorable result. In addition, this result appears to be because, unlike previous studies, the study was conducted in an Asian population, which has a lower incidence of VTE than other races19).
Potential of serious insertional injury with CCVC placement from lack of experienced personnel
In the guideline of IV catheter selection, CCVCs are the optimal choice for ICU patients who need central venous pressure (CVP) monitoring and when have an anticipated IV period of ≤ 14 days9). However, the benefit is only achieved when CCVC placement is performed by a skilled operator9).
Subclavian vein catheterization has several advantages over other CCVCs. Subclavian catheters cause less discomfort to patients, and has a lower risk of thrombosis and CLABSI than internal jugular and femoral vein catheters3,20-22). Nonetheless, subclavian catheterization has safety issues, which are mostly related to technical difficulties for identifying the target vein and subsequent mispuncture. A prospective randomized study comparing ultrasound-guided subclavian catheterization with the landmark technique reported complications such as hematomas resulting from arterial puncture (5.4%), hemothorax (4.9%), pneumothorax (4.9%), nerve plexus injury (4.3%) and cardiac tamponade (0.5%) in the landmark-guided insertion group23). These complications occur more frequently among inexperienced practitioners3,20,21). Ultrasound-guided CCVC placement has become a solution to these complications and increase catheterization success rate; it has already become a standard practice22,23).
However, ultrasound-guided catheterization is also difficult and unfamiliar to novice practitioners23). Appropriate simulation-based training and certification of techniques for ultrasound-guided vascular access have been suggested24). And the need for a training program with a consensus was also raised24). It is clear that appropriate training could reduce the complications of CVC catheterization15,22). However, whether such an educational program is well prepared at every institution is questionable. Furthermore, it seems difficult to become skilled while continuing clinical practice, considering the decrease in training time for the current residency program.
As an alternative method, puncture of the femoral or jugular vein with ultrasound guidance is much easier than puncture of the subclavian vein3,20,21). However, femoral vein catheterization has a higher risk of infection and thrombosis, which precludes maintenance for more than 4 days22). Jugular vein catheterization also has the disadvantage of strong patient discomfort, with a relatively high incidence of thrombosis and infection than subclavian catheterization20-22). The first-attempt success rate of MC insertion in our study was 96.5%. And no insertional injury was found in the MC group of our study. These outcomes which are superior to the CCVC group in our study, demonstrate the safety of MC placements with ultrasound-guided techniques even performed by residents.
Special consideration of IV infusates for neurosurgical ICU patients
Guideline recommend that infusates administered via MC should “ideally” be in the physiologic range of pH and osmolarity, which are compatible for peripheral infusion10). However, a range of pH and osmolarity of infusates and the list of medications with absolute necessities for central venous catheter (CVC) administration is not clearly established and are changing9). Although this lack of evidence, guideline have been used as a clinical reference suggesting medications for CVC rather than peripheral IV catheter or MC10). Amiodarone > 2mg/mL as an acidic agent, dextrose > 20% in non-emergent situations, hypertonic saline, total parenteral nutrient, vesicants, calcium chloride, epoprostenol, potassium concentrate > 0.1mEq.mL and vasopressors require CVC infusion10). Other medications can be administered via MCs10).
Mannitol is frequently administered for neurosurgical ICU patients. In our institution, we use 15% mannitol with an osmolarity of 823 mOsm/L (Baxter, Deerfield, IL, USA), which is lower than the 20% (1369 mOsm/L) osmolarity of definite irritants in a recent study25). Administration of hypertonic saline through a CVC is also preferred10). However, access may be limited in urgent situations for IICP control25). Recent studies have shown an acceptable safety profile for peripherally administered hypertonic saline even with bolus injection26,27).
Vasopressors induce local vasoconstriction and elevation of hydrostatic pressure, especially in small veins, and subsequently disintegrate adjacent tissues25). This potential harm is particularly concerning with respect to the accumulation of drugs in peripheral IV administration because the venous flow in the peripheral vein is slower than that in the central vein25). A meta-analysis of adverse events resulting from peripheral infusion of vasopressors showed no significant difference in complications between peripheral IV and CVC28). However, when vasopressor infusion for ≥ 24 hours or a dose increase was anticipated, a switch to CVC was recommended28). In our study, only one patient underwent norepinephrine administration via the MC; however, this patient in a few days of end-of-life caused by massive intracranial hemorrhage. In our study, vasopressors use was significantly more in the CCVC group than in the MC group (p=0.005). Antineoplastic agents classified as definite vesicants were not used in our study.
Minor complications of MC during the management period
Minor complications of MCs can result in loss of catheter function12). In a retrospective study of 411 MCs and 282 CVCs, the rate of loss of catheter function was higher in the MC group than in the CCVC group (p=0.03)29). These minor complications are not uncommon in patients with MCs12,14). A retrospective study of 115 MC placements reported that minor complications including dislodgement, kinking, skin infiltration, catheter occlusion, and thrombophlebitis occurred in 23.5% of the patients12). Although minor complications are not fatal, they should not be ignored because they can induce device removal before the completion of therapy12). However, in our study, the minor complication rate was only 5%, markedly lower than that reported previously.
Suggested MC indications for neurosurgical ICU patients
MCs are now a widely accepted device for patients with difficult IV access who have need of 5 to 14 days of administration of peripherally compatible infusate with a simple regimen10,14,30). Central venous access has clear advantages such as the capabilities of infusion of all types of medications, large volumes of blood transfusion/fluid, CVP monitoring, and complex IV therapies10). However, inserting CVCs to all neurosurgical ICU patients may be considered overtreatment and expose patients to potential harm. CCVC placements should be performed on highly selected patients; critically ill patients requiring central venous pressure monitoring for unstable hemodynamic conditions, multiple IV lines and anticipated significant blood loss on major surgery are appropriate candidates. PICC has an exceptional IV maintenance period with most advantages of CCVCs9). PICC has a certain role in the care of neurosurgical ICU patients11). However, PICC is preferred to MC if the expected IV period is ≥15 days9).
In our study, the total complication rate was significantly lower (p=0.007) in the MC catheterizations performed by residents, and the rate of completion of therapy was significantly higher than that in CCVC catheters placed by residents (p=0.002). These results ascertain that CCVC is not necessary for patients without the definitive indications described above considering the risk of CCVC-related complications.
Limitations
This study has inherent biases owing to its retrospective nature and the small sample size. In addition, the catheter type was selected by the attending physician and was not a protocol-based decision. The use of MC and PICC in neurosurgical ICU patients was initiated by an endovascular neurosurgeon not dedicated to neurocritical care. Further, not all attending staff participating in the study understood or agreed with the catheter selection rationale. The dressing methods or materials, which could have influenced the incidences of BSI and phlebitis, were not properly addressed and recorded. A well-established surveillance for DVT was not available during the study period. Our results on local practice with the above limitations may not be applicable to high-quality practice centers with experienced personnel and abundant equipment. However, our results of MC placements demonstrated a higher first-attempt success rate (96.5%) and a higher rate of therapy completion (86.0%) compared to the results of a previous study (83.4% and 80.9%, respectively)12). Our study is noteworthy because to our best knowledge, this is the first study to show the acceptable safety and efficacy of ultrasound-guided MC placement by neurosurgical residents.
CONCLUSION
CCVC placement should be decided carefully and performed in patients with a definitive need for central venous access, especially when placement is performed by a resident. MC placement by a neurosurgical resident demonstrated a significantly lower complication rate than CCVC placement by neurosurgical residents. MC placement by a neurosurgical resident may be a substitute for CCVC in neurosurgical ICU patients with a requirement of 6 to 14 days of IV therapy with a simple regimen.
Notes
Ethics statement
All study procedures involving human subjects were performed in accordance with the ethical standards of our Institutional Review Board (No. SGPAIK 2023-08-013) and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Author contributions
Conceptualization: SYC. Data curation: All authors. Formal analysis: SYC. Methodology: HJK, SYC. Project administration: SYC. Visualization: SYC. Supervision: SYC. Writing – original draft: HJK, SYC. Writing – review & editing: SYC.
Conflict of interest
There is no conflict of interest to disclose.
Funding
None.
Data availability
None.
Acknowledgements
None.