Central Pontine Myelinolysis After Non-Aneurysmal Perimesencephalic Subarachnoid Hemorrhage: Case Report and Review of the Literature
Article information
Abstract
Central pontine myelinolysis (CPM) is one of the encephalopathy that results from extreme fluctuations in serum sodium concentration and plasma osmolality. CPM after non aneurysmal perimesencephalic subarachnoid hemorrhage (NPSAH) is very rare. A 53-year-old female patient aggravated her instabilty 3 weeks after treatment of after NPSAH. Brain CT showed a prominent low-density lesion in the central pons. Vasospasm, pontine infarct, multiple sclerosis must be excluded after subarachnoid hemorrhage. Her brain magnetic resonance imaging (MRI) of the brainstem revealed CPM. The peripheral fiber sparing, central trident appearance was observed. Peripheral fiber sparing is more prominent, but central trident is disappearing at long-term follow-up MRI. CPM can develop even after NPSAH as well as aneurysmal subarachnoid hemorrhage. Trident pattern in pons area and peripheral fiber sparing is differential diagnosis with vasospasm, cerebral infarct and multiple sclerosis after NPSAH.
INTRODUCTION
Central pontine myelinolysis (CPM) was first described in 1959 by Adams and colleagues as a disease affecting alcoholics and malnourished people11). It is characterized by a loss of oligodendrocytes and myelin with relatively well preserved neuronal cell bodies and axons in the central pons9). CPM following after subarhanoid hemorrhage (SAH) caused by extreme fluctuations in serum sodium. However, CPM due to non-aneurysmal perimesencephalic SAH (NPSAH) is very rare. Here, we report and describe CPM after NPSAH.
CASE REPORT
A 53-year-old woman known to have asthma and hypertension was admitted to our emergency department with confused mentality. Initial brain computed tomography (CT) revealed SAH at the bilateral sylvian cisterns, basal cistern, and cortical sulci (Fig. 1A). Computed tomography angiography and transfemoral cerebral angiography were performed to rule out cerebral aneurysm. Neither aneurysmal dilatation nor vascular malformation was found even after repeated angiography (Fig. 1B).

A) Initial brain CT revealed SAH at the bilateral sylvian cisterns, basal cistern, and cortical sulci. B) No evidence of aneurysm, vascular malformation, or vasospasm was detected by CTA or transfemoral cerebral angiography. C) SAH at the bilateral sylvian cisterms, basal cistern, and cortical sulci resolved by the time of hospital discharge 3 weeks later. D) However, follow-up brain CT 3 weeks after discharge showed a prominent low-density lesion in the central pons. E) and F) Symmetric hyperintense lesion in the central pons with sparing of the periphery on T2-weighted MRI and hypointensity on the T1-weighted image, suggesting central pontine demyelination. G) and H) Follow-up MRI one year later showed improvement in central pontine demyelination. Peripheral fiber sparing is more prominent at long-term follow-up MRI.
The patient had severe hyponatremia (113 mmol/l) with low plasma osmolality (248 mOsm/kg). Urine sodium (25 mEq/l) and urine osmolality (201 mOsm/kg) were high. She was diagnosed with cerebral salt wasting (CSW) and was administered 3% hypertonic saline, which caused her serum sodium level to rise to 134 mmol/l within 48 hours.
When she was discharged from the hospital 3 weeks later, follow-up brain CT revealed that the SAH had resolved without CPM (Fig. 1C) and the patient was mentally alert without any neurologic symptoms. On clinical examination, her pulse rate was 72 beats/min, and her blood pressure was normal (110/70 mmHg). Her blood parameter including oxygenation, liver and renal tests were within normal limits. Her serum sodium was 136 mmol/L. She exhibited normal power in all four limbs, and her touch and pain sensation was normal.
Three weeks after discharge, however, the patient had a tendency to sway to one side when walking. She did not present dysphagia, but she had dysarthria. Brain CT showed a prominent low-density lesion in the central pons (Fig. 1D). Magnetic resonance imaging (MRI) of the brainstem revealed CPM, with a hyperintense lesion in the central pons on T2-weighted MRI and hypointensity on the T1-weighted image (Fig. 1E, 1F).
The patient was managed conservatively in outpatient care. Follow up one-year brain MRI showed some recovery brain stem lesion (Fig. 1G, 1H). Follow up one-year brain MRI showed some recovery brain stem lesion. Her clinical status was much improved; she can walk independently and returned to her daily job.
DISCUSSION
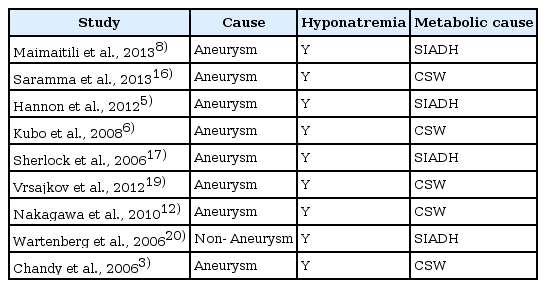
CPM after NPSAH is a very rare complication, with only a few reported cases to date. Hyponatremia is a common finding in acute brain disease, occurring in approximately 30% of neurosurgical patients with SAH, equal frequency after non-aneurysmal SAH and aneurismal SAH13). In our case, the hemorrhage was located at the bilateral sylvian cisterns, basal cistern, and cortical sulci. We have not found any aneurysmal dilatation. A falling sodium level can progressively lead to neurological problems such as confusion, lethargy, seizures, and coma2). CPM has traditionally been associated with rapid correction of hyponatremia, allcoholism, chronic malnutrition, and sodium imbalance are the primary conditions associated with CPM1). Although early case reports described CPM associated with alcoholism10), more recent studies have implicated rapid corrections of hyponatremia and hypernatremia as a cause of CPM or Osmotic demyelination syndrome (ODS)7). Reported cases in central pontine demylination after aneurysamal or non aneurysmal subarachnoid hemorrhage shown in Table 1.
Specifically, clinical studies suggest that rapid correction of hyponatremia, especially a large magnitude of correction (i.e., >25 meq/l in the first 24-48 hours) is associated with CPM18). As excessive natriuresis has been observed, the term CSW was coined for this syndrome. However, the proposed natriuretic factor was not identified, and after the discovery of antidiuretic hormone (ADH), the syndrome of inappropriate ADH secretion was favored as the causal mechanism2).
The preferential localization of myelinolysis to the central pons is thought to result from the grid arrangement of oligodendrocytes, which limits their mechanical flexibility and capacity to swell. Oligodendroglial cells are vulnerable to cellular dehydration, which may enhance apoptosis4). During hyponatremia, these cells can only adapt by losing more ions instead of swelling, making them prone to damage when sodium is replaced. Central trident sign and peripheral fiber sparing was observed at initial MRI, and peripheral sparing more prominent at long-term follow up MRI in this case.
ODS, which includes CPM and extrapontine myelinolysis, is evident on CT. However, MRI is the imaging technique of choice due to its greater sensitivity and superior capacity to detect the lesions. The typical radiologic feature of CPM is a trident-shaped, symmetric, hyperintense lesion in the central pons with sparing of the periphery on T2-weighted MRI and hypointensity on the T1-weighted image15). Diffusion-weighted imaging (DWI) with apparent diffusion coefficient mapping yields higher specificity for ODS lesions with early signal changes after the onset of neurologic symptoms14).
When osmotic demyelination occurs, it is usually irreversible and has no definitive management. Thus, prevention is more important. Indeed, the slow correction of hyponatremia does not appear to be associated with ODS either clinically or experimentally. Reports of individual cases or small case series suggest that treatments including steroids, intravenous immunoglobulin, and thyrotrophin-releasing hormone may have good outcomes, but these results have been difficult to interpret.
CONCLUSION
In conclusion, CPM can develop not only after aneurysmal SAH with thick subarachnoid hemorrhage but also after NPSAH. Brain MRI image showing brain stem lesion, and peripheral sparing can be detected after CPM at follow up MRI. Trident pattern in pons area and peripheral fiber sparing is differential diagnosis with vasospasm, cerebral infarct and multiple sclerosis after non-aneurysmal perimesencephalic subarachnoid hemorrhage.
Notes
No potential conflict of interest relevant to this article was reported.