INTRODUCTION
Arteriovenous malformations (AVM) is a congenital vascular abnormality with arterial and venous shunts. Incidence of AVM is 1.21/100,000 personŌĆæyears (95% confidence interval 1.02ŌĆō1.42)1). Posterior fossa AVMs are rare, consisting 7ŌĆō15% of intracranial AVMs2). Hemifacial spasm (HFS) is a disease characterized by unilateral involuntary tonic and clonic contraction of the facial muscles. HFS are caused by normal vascular structure compressing the root exit zone of the facial nerve. Such vascular compression mainly results from by nearby arteries. HFS can occur secondarily by intracranial pathology, such as cerebellopontine angle tumor or vascular abnormality like AVM or aneurysm3). Microvascular decompression (MVD) is the most effective treatment of option for HFS, as MVD offers the overall spasm-free rate over 90%4).
Neurovascular compression syndrome such as HFS or trigeminal neuralgia related to AVM is very rare, and 37 cases have been reported so far5). Herein, we report a case of HFS patient with cerebellar AVM. The symptom of HFS was relieved after surgical removal of AVM nidus without MVD of the facial nerve.
CASE REPORT
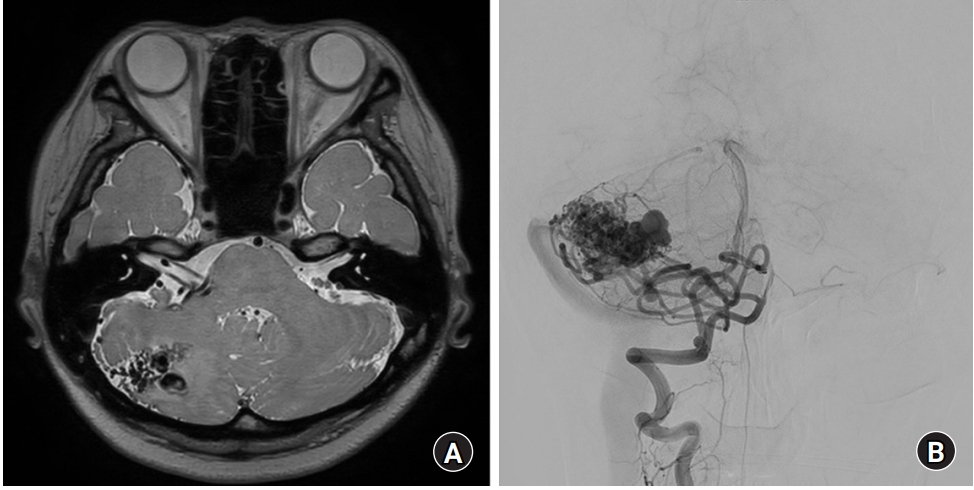
A 40-year-old woman visited the outpatient clinic for right hemifacial spasm. The symptom started 7 years ago. Her initial symptom was involuntary periocular muscle contraction, which was aggravated by stress and anger. The symptom gradually spread to perioral muscle after 5 years of initial symptom, and the severity and the frequency of facial spasm were increased. She experienced no response to other conservative management such as medication, and refused botulinum toxin injection due to its temporary effect. Neurological examination revealed right tonic and clonic facial spasm involving both periocular and perioral regions. Magnetic resonance imaging (MRI) showed an abnormal vasculature with maximum diameter of 3 cm in the right cerebellar hemisphere, which was suspected to be an AVM. Right anterior inferior cerebellar artery (AICA) and posterior inferior cerebellar artery (PICA), together with prominent venous structure, compressed the root exit zone of the ipsilateral facial nerve (Fig. 1A). Conventional cerebral angiography showed a right cerebellar AVM supplied by right PICA and AICA, and drained into sigmoid and occipital sinus (Fig. 1B).
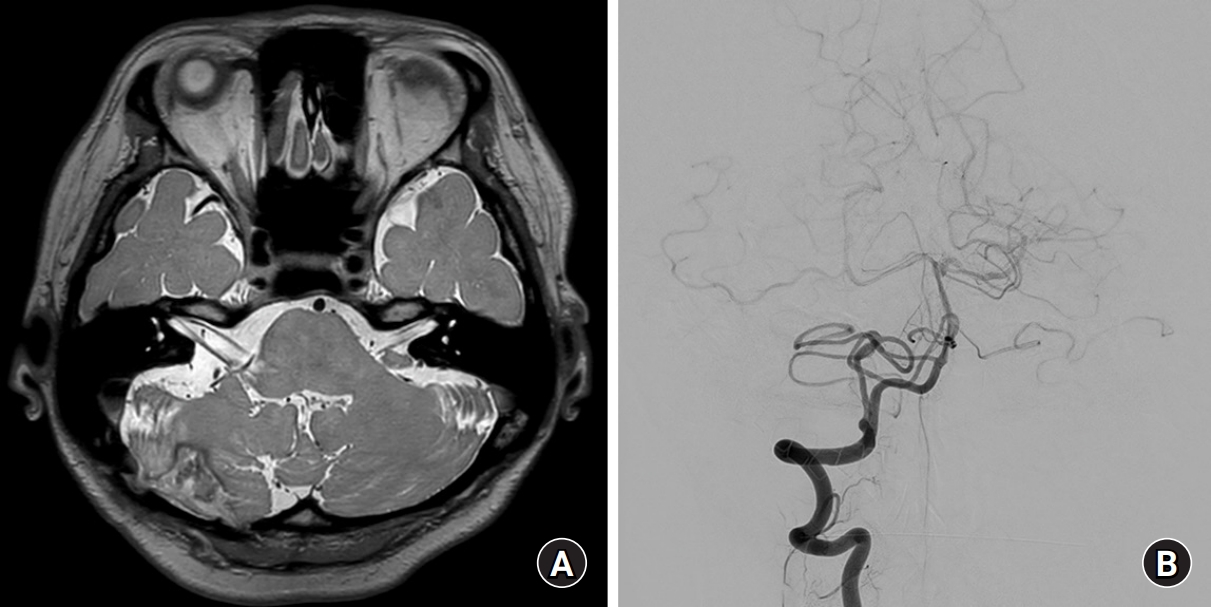
The patient underwent a successful nidus removal without MVD of the facial nerve. Postoperative MRI taken 6 months after surgery showed that the diameters of the offending vessels around the facial nerve were significantly decreased (Fig. 2A). Conventional angiography was followed up after the surgery and showed no evidence of residual AVM. Early draining veins were not observed, and the flow and size of feeding arteries slowed and decreased, respectively (Fig. 2B). The symptom of HFS was much relieved after 6 months of surgery, and hardly observed after 2 years of surgery.
DISCUSSION
The risk of intracranial hemorrhage in cerebellar AVM is reported to be 2ŌĆō4% per year, with a mortality rate of 18%6,7). It is one of the most common cause of intracranial hemorrhage, especially in young adults8,9). Although there is still controversy about the timing and method of treatment for unruptured AVM, emergent surgery is required in case of ruptured AVM. And intraventricular hemorrhage or hydrocephalus can be rapidly accompanied in ruptured AVM, which can also put the patient at great risk. On the other hand, HFS is not a life-threatening disorder and the treatment for HFS can be delayed. Moreover, the root exit zone of the facial nerve could not be exposed due to abnormal vasculature all along the cerebellopontine angle in this case, and the compressing vessels of our patient might be shrunk and give less effect on the facial nerve after the appropriate treatment of AVM. Therefore, we decided to treat the AVM first. And, as in prior case reports where removal of AVM was performed without MVD5), the symptom of HFS in our patient is almost completely relieved for 2 years after surgery.
AVM caused by neurovascular compression syndrome, such as HFS or trigeminal neuralgia, is very rare. Among 37 cases, which have been reported till now, only 7 cases were found to be involved with HFS. Treatments for HFS caused by AVM were varied. Craniotomy and AVM removal or endovascular intervention with or without MVD were provided, and in terms of spasm relief, the outcome was successful regardless of the combination of treatment options.
In our case, the symptom of HFS was much improved after AVM removal without MVD. It is postulated that the changes in hemodynamics of vasculature around the root exit zone of the facial nerve resulted in decreased physical compression on the nerve, and thereby might yield physiological restoration of the nerve. If the symptom of HFS in our case recur in the future, we will consider MVD surgery. This case report does not include any individually identifiable health information, number, characteristic, or code to identify patients, and carries no risk for the patients. This study was therefore exempt from institutional review board approval.
CONCLUSIONS
Here, we presented the case of HFS caused by AVM that HFS symptom disappeared after AVM removal without MVD. HFS caused by AVM is rare and optimal combination of treatment options has not been established. If performing AVM treatment and MVD at the same time is too risky, it is recommended to do AVM treatment first and wait for HFS symptom relief.